Distillation Process and Applications in Food Industry
Distillation is a separation process used in the food industry to separate components in a mixture based on their volatility. It involves vaporization, condensation, and re-evaporation to achieve separation. Steam distillation is a method where steam is used to reduce boiling temperatures for safe separation of volatile materials from non-volatile ones in food processing applications.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
DISTILLATION PROCESS AND APPLICATION IN FOOD INDUSTRY Food Engineering (DTE 321) Dr. Jahangir Badshah University Professor-cum-Chief Scientist Dairy Engineering Department, SGIDT, Patna (Bihar Animal Sciences University, Patna)
Distillation Process Distillation is a separation process, components in a mixture by making use of the fact that some components vaporize more readily than others. separating When vapours are produced from a mixture, they contain the components of the original mixture, but in proportions which are determined by the relative volatilities of these components.The vapour is richer in some components which are more volatile,and so a separation occurs.
Distillation Process In fractional distillation, the vapour is condensed and then re-evaporated when a further separation occurs. It is difficult and sometimes impossible to prepare pure components in this way, but a degree of separation can easily be attained if the volatilities are reasonably different. Where great purity distillations may be used. is required, successive
Steam Distillation A liquid boils when the total vapour pressure of the liquid is equal or more than the external pressure on the system. Therefore, boiling temperatures can be reduced by reducing the pressure on the system; for example by boiling under a vacuum, or by adding an inert vapour which by contributing to the vapour pressure, allows the liquid to boil at a lower temperature. Such an addition must be easily removed from the distillate, if it is unwanted in the product, and it must not react with any of the components that are required as products. The vapour that is added is generally steam and the distillation is then spoken of as steam distillation.
Steam Distillation In some circumstances in the food industry, distillation would appear to be a good separation method but it cannot be employed temperatures would lead to breakdown of the materials. In cases in which volatile materials have to be removed from relatively non-volatile materials, steam distillation may sometimes be used to effect the separation at safe temperatures. directly as the distilling
Principle and applications of steam distillation If the vapour pressure of the introduced steam is psand the total pressure is P, then the mixture will boil when the vapour pressure of the volatile component reaches a pressure of (P - ps), compared with the necessary pressure of P if there were no steam present. The distribution of steam and the volatile component being distilled,in the vapour,can be calculated. The ratio of the number of molecules of the steam to those of the volatile component, will be equal to the ratio of their partial pressures. Pa/ps= (P-ps)/ps= wa/Ma/ws/Ms
Principle of distillationcontd. The weight ratio wa/ws= (P-ps)/psx(Ma/ Ms) Application in the preparation of some volatile oils and in the removal of some flavours from edible fats and oils. Higher the molecular weights of volatile components, lower will be the requirement of steam in weight.
Types of Distillation Equipments Batch Distillation or flash distillation a. Effective for separating components that boil at widely different temperatures. b. It requires many successive re-distillations to get pure components,which is inefficient. Vacuum Distillation or Batch Distillation under vacuum i. When the vapour pressure of volatile components reaches the vacuum,the distillation occurs. ii. Distilling at lower temperatures for heat sensitive materials iii. Vacuum steam distillation combine the two methods and are more suitable equipments. in modern distillation
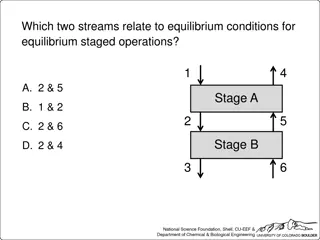
Continuous Distillation with fractional column Continuous Distillation with Reflux having fractional column for rectification and stripping It consists of a boiler, a column in which actual contact stages for the distillation separation are provided and a condenser for condensation of the final top products. Liquid feed flows down the column to the still or reboiler and is subjected to rectification by the vapour rising from the reboiler. Rectification in lower section of the column is called stripping. The conventional arrangement in fractionating column is in the form of bubble cap trays. The vapour rise through the bubble caps and the liquid from reflux line past the bubble caps where it contacts the vapour, and then passes over a weir down to the next trays. Each tray represents a contact stage, where some of the more volatile component of liquid ( as component A) is vapourized and some of the less volatile component from vapour (as component B) is condensed and seaparation occurs till equilibrium. In steam distillation, steam is bubbled through the liquid and vapours containing the volatile component and steam is passed to the condenser. In some cases, the steam and the condensed volatile component are immiscible,so the separation in the condenser is simple.
Applications of Distillation Extraction of Essential oils from leaves, seeds: Use of Steam distillation in fractional distillation either in normal pressure or vacuum. Recovery of solvent from oil after extraction: Use of falling film evaporators for less viscous oil is preferred for evaporation.The last traces of solvent in the viscous or concentrated oil may be removed by steam distillation. Concentration of Aroma compounds from Juice and extracts: Use of vacuum evaporator for collecting volatile aroma compounds in vapour followed by distillation column and condenser to collect aroma in liquid state.
Applications in beverages preparation Manufacture of Whisky (a spirit): Produced from distillation of a mash of cereal including barely, corn, rye and wheat. Scotch and Irish whisky: Malt whisky produced from100 % malted (germinated) barely Grain Whisky produced from unmalted cereal grains Blended whisky: Containing 60 -70 % grain whisky and 30- 40% malt whisky. Alcohol boils at a lower temperature than water, and therefore will evaporate first. It is separted off into another container called condenser. The wash is trickled down a series of plates with steam rising up in opposite direction. The steam heats the coil as it goes and it evaporates off.
Manufacture of Neutral Spirit A multicolumn producing neutral spirits from fermented mash of cereals. The fermented mash containing 7 % (v/v) of alcohol is fed to near the top of the whisky separating column. The overhead distillate from this column is fed to aldehyde column. The bottom product from this column is pumped to the middle of the product concentrating column. The end product, neutral spirit, is withdrawn from near the top of this column. distillation plant is used for
Thank You ejazbadshah@gmail.com