Mixture Separation Lab Procedure & Analysis
In this practical lab activity, students are tasked with separating a mixture containing Sand, Salt, Poppy Seeds, and Iron Filings. The procedure involves a step-by-step approach including identifying three separation strategies, executing the chosen method, recording observations, and calculating percent compositions and recoveries. Through qualitative and quantitative data collection, participants aim to effectively isolate and quantify each substance in the mixture.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
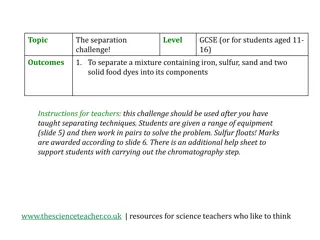
Separation Lab overview Given a mixture of Sand, Salt, Poppy Seeds and Iron Filings, design and execute a separation method. Write a proposed procedure to start. When executing your procedure, record every action you take in the order you do them to create the real procedure that you used. Separate, dry and weigh each of the four substances. Determine the percent composition of each of the four substances in the mixture. Determine the percent recovery of matter from the mixture.
Separation strategies Basic strategy was to separate out the iron first using a magnet, then use water to help separate the other three. Most of you used the first strategy. Some of you filtered the poppies with the sand and had to add a second portion of water and decant. (If you added water before using the magnet, you d use the last strategy.) Identify which of the three strategies you used. A, B, or C A. B. C.
Abbreviated Report Format Shortened format (still 25 pts) not all sections of the general report present. Perform lab work in pairs or trios Share data with partner(s) Each student writes their own report Hand written is easiest for this report. But typed is acceptable. Prelab (3) Include your original flow chart, procedure and data table. Procedure (5) Include the notes you generated during lab and any measurements you made. Indicate which of the above strategies you used, A, B, or C. Data Table and Calculations (10) four dry mass weights, percent compositions, percent recovery Discussion questions (7) from lab handout
Procedure KEY for this lab!!! Attach your first proposed procedure as your prelab List your ACTUAL procedure in the report Describe what YOU ACTUALLY DID!! If you didn t write it down at the time, you may recreate your procedure from memory, but DO NOT copy a procedure from someone else. Should be different from your proposed procedure as you tried things and made modifications based on observations Should include the specific kinds of equipment you used (eg. 250 mL beaker)
Data and Calculations You should have plenty of qualitative observations for this lab, recording what things looked like after significant steps in the procedure. The quantitative data you should have will be what you really put on a balance: Mixture + cup, Poppy + filter paper, Filter paper, or empty beaker etc Calculations: Determine the initial mass of the mixture. Determine the mass of the separated sand, salt, iron and poppy. Determine the total mass recovered. Determine the percent composition of each of the 4 substances. (component/mixture x 100) Determine the percent recovery. (sum of 4 components/mixture x 100)
Questions For each of the four components, describe a specific physical property that enabled you to separate it from the rest of the mixture. In your estimation, how successful were you (on a scale of 1-10) in separating and recovering each of the four components: Sand, Salt, Fe Filings, and Poppy Seeds? Justify your estimations of your success, based on your observations. What made you decide to do your procedural steps in the order in which you did them? Would any order have worked? If you were able to do the lab over again, what specific things would you do differently? Name two materials or tools that weren t available that might have made your separation easier.