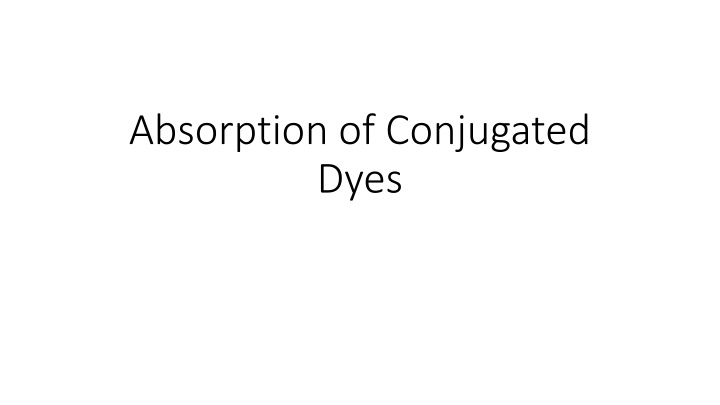Analysis of Absorption Properties of Conjugated Dyes
The analysis focuses on the absorption properties of conjugated dyes such as 1,1-diethyl-2,2-cyanine iodide (red), 1,1-diethyl-2,2-carbocyanine iodide (blue), and 1,1-diethyl-2,2-dicarbocyanine iodide (turquoise). It involves calculating lambda max for each dye based on a particle in a box model, comparing literature/experimental and calculated lambda max values, and determining the energy corresponding to the experimental electronic transition. Additionally, the length of the box is calculated using the provided equations.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Organic dyes (1) 1,1 -diethyl-2,2 -cyanine iodide (red) (2) 1,1 -diethyl-2,2 -carbocyanine iodide (blue) (3) 1,1 -diethyl-2,2 -dicarbocyanine iodide (turquoise) (cyanine) (carbocyanine) (dicarbocyanine) Dye Preparation Stock solutions of the dyes are individually prepared using methanol. Further dilutions are made until an absorbance close to 1.0 is obtained.
Data max, nm Dye Concentration, M Absorbance 1.1 x 10-4 (1) Cyanine 0.891 523.0 8.7 x 10-6 (2) Carbocyanine 0.728 604.0 4.1 x 10-6 (3) Dicarbocyanine 0.760 706.5
Analysis 1. 2. 3. 4. 5. Calculate the lambda max for each dye based on particle in a box model (eqn 6 in the book reference). Compare the literature/experimental and calculated lambda max. Compare the literature and experimental absorption coefficient (path length is 1 cm). Calculate the energy corresponding to the experimental electronic transition. Calculate the length of the box (use equation in the book reference).
Procedure The pulse width, relaxation times, and optimum pulse angle of an ethyl benzene in deuterated chloroform solution are measured. The procedure for the NMR analysis is uploaded separately in a word file. Step 1: In this step, a normal NMR procedure is run to verify the purity of the ethyl benzene. First, the sample is loaded into the NMR, autolocked in CdCl3 solvent, then shimmed to generate homogeneous magnetic field. The experiment was set to a single pulse, 90 degree pulse angle, and 25 s relaxation delay. The optimum receiver gain, which is a parameter to maximize the signal to noise ratio is obtained in this step. Step 2: The goal of this step is to measure the 180 degree pulse width. Similar procedure in step 1 is followed with some modifications: a. Unchecking the autogain and setting the gain to the value obtained in step 1. b. Pulse width in linear mode: Starting value is 5 us, End value is 35 us, and Increment is 5 us.
Procedure Step 3: The goal of this step is to measure the T1 (spin-lattice relaxation time) value by the inversion recovery method. Instead of a single pulse, a double pulse experiment is employed. The receiver gain value obtained from Step 1 and the 90 degree pulse width value obtained from Step 2 are used. The value of tau is arrayed exponentially starting at 0.05 s and ending at 25 s. After acquisition the data is processed individually for all peaks (three graphs). Step 4: The goal of this step is to measure the optimum flip angle for 3 values of D, where D = AT + dl (D is time domain, AT is acquisition time, and dl is relaxation delay). The receiver gain value obtained from Step 1 and the 90 degree pulse width value obtained from Step 2 are used. Acquisition time is set to 1s while the relaxation delay is varied for each run (1s, 2s, and 4s; hence D=2s, 3s, and 5s, respectively for each run). In each run, the pulse angle was arrayed starting at 30 degree, ending at 90 degree, and an increment of 15 degree. One of the three peaks was chosen for analysis to compare the three D values.
Data and Analysis All the data is in a pdf file with a title NMR data . Step 1: You should have the spectrum with peaks assigned and coupling determined. Receiver gain value is highlighted. Step 2: You should have a graph of a weighted linear inversion recovery. The value (x-axis value) where the y-value crosses zero is the 180 degree pulse. Dividing this value by 2 will give the pulse width for 90 degree pulse. Step 3: You should have three printed T1 data for all 3 peaks of ethyl benzene (T1 value and peak analyzed is boxed in red). Compare the values obtained to those in the literature. You should explain pictorially why the signal appears upside down for short tau values and right side up for longer tau values. Determine the total delay (AT + dl) at which each of the peaks will assume 95% of their maximum value in a single pulse experiment using a 90 degree pulse. To answer the last question check the uploaded file with the title Optimum Pulse Width and Recycle Delay in Single Pulse Experiment . Step 4: You should have three plots for all the D values (D value for each plot is written in the right side). Determine the pulse angle (maximum signal) for each plot. In your data, you should provide a plot of optimum pulse angle vs D. Only one peak is considered, do you expect the same optimum flip angle for each of the peaks in your spectrum?
Set-up and Procedure 1. One gram of NaCl is loaded into a three-neck rbf. One neck is capped, the second neck is connected to a solvent reservoir (which is capped for gas not to escape), and the third is connected to a tube (which is connected to a gas cell). 2. The solvent reservoir is filled with 1 mL of D2SO4, while the stopcock is closed. 3. D2SO4 is then added all at once and bubbles are produced in the reaction. Initially, stopper 2 is loosened so that any trapped gases from air can be removed from the collection vessel. After few seconds, stopper 2 is tightened to contain the HCl/DCl gases. 4. When the bubbles cease to form, stopper 1 is also tightened and the tube is disconnected from the rbf. 5. The gas collection vessel is then analyzed in IR. 6. Background with just air is run, then the vessel was inserted for analysis. 7. Data for IR are in separate files with the titles, HCl_DCl data and HCL DCl . Filled with D2SO4 Filled with NaCl Stopper 1 Gas collection vessel Stopper 2
Analysis Refer to the book reference
Procedure A selenium stock solution (Stock A) is prepared by adding 60 mg Se, 10 mL octadecene, and 0.8 mL trioctylphosphine to a 50 mL rbf. At this point, the mixture is heterogeneous having black solid suspended in a colorless liquid. The mixture is stirred and heated (~60 C) for 15 minutes until the selenium dissolves and forms a colorless solution. The solution is then cooled to RT. In a 100 mL rbf, 25.5 mg cadmium oxide (red granule) , 20 mL octadecene, and 1.2 mL of oleic acid (Stock B) are mixed then heated. A color change from light yellow to yellow-orange is observed. The solution was heated until it reach 225 oC. Once the cadmium oxide solution reach 225 oC, 2 mL of Se stock solution is added and the timer started. When the Se solution is added, the solution turned yellow.
Procedure From the mixture, a pipette full was pulled out at various time points (10 time points) and place into a vial. Time points: 10s, 20s, 30s, 40s, 50s, 65s, 85s, 110s, 140s, 180s. Note the change in color as observed below, where the vial labelled 1 is time 0s. The samples in the vials were cooled down to RT then diluted with octadecene until an absorbance (1st peak maximum) between 0.8-1.5 is obtained from UV-vis (350-700 nm) The sample that is use for UV-Vis is transferred to the fluorimeter for analysis. Emission spectra is run at 370 nm excitation wavelength and scanned from 400-700 nm. The peak is recorded. Data obtained is in a separate file with the title QD Absorbance and QD Fluorescence .
Analysis Refer to the book reference