Effectiveness of Daclatasvir and Sofosbuvir in HCV Genotype 3: Insights from ALLY-3 Study
Study on the efficacy of Daclatasvir and Sofosbuvir in treating Hepatitis C Virus (HCV) Genotype 3, involving treatment-naive and treatment-experienced patients. The Phase 3 trial showcased promising results in achieving SVR12, with detailed patient characteristics, drug dosing, and baseline characteristics influencing treatment outcomes. Key findings include high SVR rates, favorable tolerability, and the importance of baseline factors like cirrhosis and prior treatment failure. The study, published in Hepatology 2015, provides valuable insights for managing HCV Genotype 3 infections.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Phase 3 Treatment-Na ve and Treatment-Experienced Daclatasvir + Sofosbuvir in Genotype 3 ALLY-3 Study Nelson DR, et al. Hepatology 2015;61:1127-35.
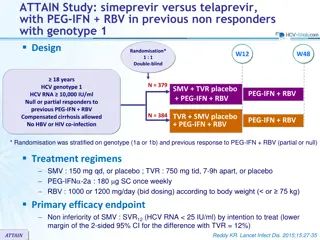
Daclatasvir + Sofosbuvir for HCV GT 3 ALLY-3 Trial: Study Features Daclatasvir + Sofosbuvir Trial: Features Design: Phase 3 open-label two-cohort study of daclatasvir (DCV) plus sofosbuvir (SOF) in treatment-na ve or experienced, chronic HCV GT 3 Setting: Multiple centers in the United States Entry Criteria - Chronic HCV genotype 3 - Treatment-na ve or treatment-experienced (prior NS5A experience excluded) - HCV RNA 10,000 IU/ml - Compensated cirrhosis allowed (METAVIR F4 on biopsy, FibroScan >14.6 kPa or FibroTest (FibroSURE) score 0.75 with APRI >2) Patient Groups - N = 101 treatment-na ve GT3: DCV + SOF x 12 weeks - N = 51 treatment-experienced GT3: DCV + SOF x 12 weeks End-Points: Primary = SVR12 Source: Nelson DR, et al. Hepatology 2015;61:1127-35.
Daclatasvir + Sofosbuvir for HCV GT 3 ALLY-3 Trial: Design Week 0 12 24 Daclatasvir + Sofosbuvir Treatment-Na ve n=101 SVR12 Treatment-Experienced n=51 SVR12 Daclatasvir + Sofosbuvir Drug Dosing Daclatasvir: 60 mg once daily Sofosbuvir: 400 mg once daily Source: Nelson DR, et al. Hepatology 2015;61:1127-35.
Daclatasvir + Sofosbuvir for HCV GT 3 ALLY-3 Trial: Patient Characteristics Characteristic Treatment-Na ve (n=101) Treatment-Experienced (n=51) Male 58 (57%) 32 (63%) Median age, years (range) 53 (24-67) 58 (40-73) Race White Black Asian 92 (91%) 4 (4%) 5 (5%) 45 (88%) 2 (4%) 2 (4%) HCV RNA 800,000 IU/ml 70 (69%) 38 (75%) Cirrhosis 19 (19%) 13 (25%) IL28B non-CC genotype 61 (60%) 31 (61%) Prior treatment failure Relapse Partial response Null response Othera N/A N/A N/A N/A 31 (61%) 2 (4%) 7 (14%) 11 (22%) a Intolerant of therapy (n=6), virologic breakthrough (n=2), HCV never undetectable on treatment (n=2) Source: Nelson DR, et al. Hepatology 2015;61:1127-35.
Daclatasvir + Sofosbuvir for HCV GT 3 ALLY-3 Trial: Results ALLY-3: SVR12, by Baseline Characteristics Status 100 94 92 91 90 88 87 86 80 70 60 SVR12, % 40 20 58/62 128/142 7/10 40/44 95/108 55/60 80/92 77/90 0 Male Female <65 <800K 800K CC Non-CC 65 Gender Age (Years) HCV RNA IL28B Genotype Note: SVR 12 based on HCV RNA less than lower limit of quantitation (25 IU/mL), detectable or undetectable Source: Nelson DR, et al. Hepatology 2015;61:1127-35.
Daclatasvir + Sofosbuvir for HCV GT 3 ALLY-3 Trial: Results ALLY-3: SVR12, by Cirrhosis Status 100 90 89 86 80 (%) with SVR12 60 40 20 91/101 44/51 135/152 0 All Patients Treatment-na ve Treatment-experienced Source: Nelson DR, et al. Hepatology 2015;61:1127-35.
Daclatasvir + Sofosbuvir for HCV GT 3 ALLY-3 Trial: Results ALLY-3: SVR12, by Cirrhosis Status No cirrhosis Cirrhosis 100 97 96 94 80 (%) with SVR12 69 60 63 58 40 20 73/75 32/34 11/19 9/13 20/32 73/75 11/19 32/34 9/13 105/109 0 All Patients Treatment-na ve Treatment-experienced Note:11 had missing or inconclusive findings for cirrhosis and not included in denominators Source: Nelson DR, et al. Hepatology 2015;61:1127-35.
Daclatasvir + Sofosbuvir for HCV GT 3 ALLY-3 Trial: Adverse Events Daclatasvir + Sofosbuvir (n=152) Event Serious Adverse Events (AEs) 1 (1%) AEs leading to discontinuation 0 Grade 3 or 4 AEs 3a (2%) Adverse Events in 10% of patients Headache Fatigue Nausea 30 (20%) 29 (19%) 18 (12%) Grade 3 or 4 Lab Abnormalities Hemoglobin < 9 g/dL Neutrophils < 0.75 x 109/L Platelets < 50 x 109/L Lipase > 3 x ULN 0 0 2 (1%) 3 (2%) aAll were grade 3 AEs. ULN = upper limit of normal Source: Nelson DR, et al. Hepatology 2015;61:1127-35.
Daclatasvir + Sofosbuvir for HCV GT 3 ALLY-3 Trial: Conclusion Conclusion: A 12-week regimen of daclatasvir plus sofosbuvir achieved SVR12 in 96% of patients with genotype 3 infection without cirrhosis and was well tolerated. Additional evaluation to optimize efficacy in genotype 3-infected patients with cirrhosis is underway. Source: Nelson DR, et al. Hepatology 2015;61:1127-35.
This slide deck is from the University of Washingtons Hepatitis C Online and Hepatitis Web Study projects. Hepatitis C Online www.hepatitisc.uw.edu Hepatitis Web Study http://depts.washington.edu/hepstudy/ Funded by a grant from the Centers for Disease Control and Prevention.