C-EDGE.TN Study: Grazoprevir/Elbasvir in Genotype 1, 4, or 6 - Treatment for HCV Infection
The C-EDGE.TN Study evaluated the efficacy of grazoprevir/elbasvir treatment in patients with HCV infection, specifically genotype 1, 4, or 6. The study included 316 patients and aimed to achieve SVR12 rates superior to historical standards. The treatment was well-tolerated, with high SVR12 rates across different subgroups, indicating the efficacy of grazoprevir/elbasvir in this patient population.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
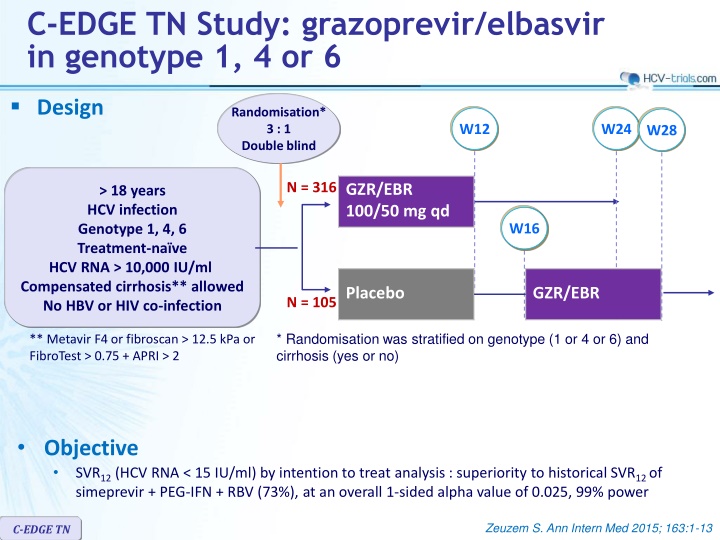
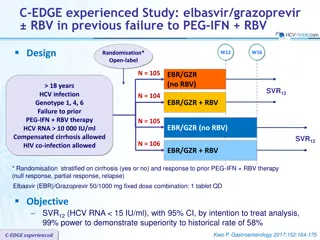
C-EDGE TN Study: grazoprevir/elbasvir in genotype 1, 4 or 6 Design Randomisation* 3 : 1 Double blind W12 W24 W28 N = 316 GZR/EBR 100/50 mg qd > 18 years HCV infection Genotype 1, 4, 6 Treatment-na ve HCV RNA > 10,000 IU/ml Compensated cirrhosis** allowed No HBV or HIV co-infection W16 Placebo GZR/EBR N = 105 * Randomisation was stratified on genotype (1 or 4 or 6) and cirrhosis (yes or no) ** Metavir F4 or fibroscan > 12.5 kPa or FibroTest > 0.75 + APRI > 2 Objective SVR12 (HCV RNA < 15 IU/ml) by intention to treat analysis : superiority to historical SVR12 of simeprevir + PEG-IFN + RBV (73%), at an overall 1-sided alpha value of 0.025, 99% power Zeuzem S. Ann Intern Med 2015; 163:1-13 C-EDGE TN
C-EDGE TN Study: grazoprevir/elbasvir in genotype 1, 4 or 6 Baseline characteristics and patient disposition GZR/EBR N = 316 52.2 46% 19% / 60% / 17% Placebo N = 105 53.8 47% 17% / 70% / 12% Age, years, mean Female Black / White / Asian Genotype 1a 1b 4 6 50% 42% 6% 3% 6.4 34% 22% 77 (62) 190 5 51% 38% 8% 3% 6.4 35% 21% 75 (64) 194 1 HCV RNA log10IU/ml IL28B CC Metavir F4 ALT, IU/L, mean (SD) Platelets < 109/L, mean Discontinuation, n For adverse event / lost to follow-up / death 3 / 1 / 1 1 / 0 / 0 Zeuzem S. Ann Intern Med 2015; 163:1-13 C-EDGE TN
C-EDGE TN Study: grazoprevir/elbasvir in genotype 1, 4 or 6 SVR12(HCV RNA < 15 IU/ml), % (95% CI) 99 100 94.6 92 % (95-100) (82-100) (91.5-96.8) 100 (86-96) 80 (44-98) 75 50 25 316 157 131 18 10 0 All patients Genotype 1a GT 1b GT 4 GT 6 Non-virologic failure 4 3 1 0 0 Breakthrough 1 1 0 0 0 Relapse 12 9 1 0 2 Zeuzem S. Ann Intern Med 2015; 163:1-13 C-EDGE TN
C-EDGE TN Study: grazoprevir/elbasvir in genotype 1, 4 or 6 SVR12(HCV RNA < 15 IU/ml) by subgroup, % (95% CI) 97 (92-99) 97 93 96 94 100 93 92 % (90-100) (87-97) (92-98) (90-97) (96-100) (88-96) (88-96) 100 75 50 25 0 94 222 171 106 208 246 70 145 No Yes Male CC Non-CC No Yes Female IL28B genotype Cirrhosis Sex HCV RNA > 800 000 IU/ml Zeuzem S. Ann Intern Med 2015; 163:1-13 C-EDGE TN
C-EDGE TN Study: grazoprevir/elbasvir in genotype 1, 4 or 6 SVR12in genotype 1 according to baseline NS3 and NS5A RAVs RAV SVR12 SVR12 SVR12 at baseline % (n/N) all patients % (N/n) RAVs with 5-fold susceptibility RAVs with > 5-fold susceptibility NS3 RAVs Genotype 1a Present Absent Genotype 1b Present Absent NS5A RAVs Genotype 1a Present Absent Genotype 1b Present Absent All 11 patients with virologic failure had baseline HCV RNA > 800,000 IU/ml (selection of NS5A RAV in 10/11) 57% (86/151) 43% (65/151) 97% (83/86) 89% (58/65) 97% (83/86) - 0% (0/0) - 19% (25/129) 81% (104/129) 96% (24/25) 100% (104/104) 96% (21/22) - 100% (3/3) - 12% (19/154) 88% (135/154) 58% (11/19) 99% (133/135) 90% (9/10) - 22% (2/9) - 14% (18/130) 86% (112/130) 94% (17/18) 100% (112/112) 100% (1/1) - 94% (16/17) - Zeuzem S. Ann Intern Med 2015; 163:1-13 C-EDGE TN
C-EDGE TN Study: grazoprevir/elbasvir in genotype 1, 4 or 6 Baseline and emergent resistance variants in virologic failure cases NS3 RAVs NS5A RAVs Baseline HCV RNA (IU/ml) 1,238,923 5,127,102 2,134,448 Day of virologic failure Rx D71 F/U D28 F/U D71 Emerging at failure (in addition) V36M None D168A Emerging at failure (in addition) Q30R Q30R Y93H GT Baseline Baseline Breakthrough Relapse Relapse 1a 1a 1a Q80K, S122G WT WT L31L/M L31M Q30H/Q M28V, Q30L, Y93H M28M/V, H58H/D L31M WT WT Y93H Q30R, L31L/M Relapse 1a 948,279 F/U D70 Q80K D168A L31V Relapse 1a 3,908,965 F/U D84 WT D168A Q30R Relapse Relapse Relapse Relapse Relapse 1a 1a 1a 1b 1a 5,282,871 1,846,427 1,939,436 4,475,338 3,913,374 FU D62 F/U D54 F/U D61 F/U D89 F/U D29 WT WT WT T54S V55A None None Q30R Q30R, L31M Y93N L31F None Y56H, D168A V170I D168A V36I, L80Q, S122T, I132L, I170V WT L80K, I170V Relapse 6 15,689,194 F/U D25 D168Y WT None Relapse Relapse 1a 6 1,574,151 15,056,901 F/U D56 F/U D25 None M28V F28L M28G L31M Y56Y/H, D168E Zeuzem S. Ann Intern Med 2015; 163:1-13 C-EDGE TN
C-EDGE TN Study: grazoprevir/elbasvir in genotype 1, 4 or 6 Adverse events, n (%) GZR/EBR N = 316 Placebo N = 105 Common AEs (> 5% in GZR/EBR) Headache Fatigue Nausea Arthralgia 52 (17%) 49 (16%) 28 (9%) 20 (6%) 19 (18%) 18 (17%) 8 (8%) 6 (6%) Non-cirrhotic Cirrhotic GZR/EBR N = 246 Placebo N = 83 GZR/EBR N = 70 Placebo N = 22 At least one adverse event Drug-related adverse event Serious adverse events Serious drug-related adverse event Discontinuation due to adverse event Death 175 (71%) 96 (39%) 7 (3%) 0 (0%) 2 (1%) 1 (<1%) 57 (69%) 32 (39%) 3 (4%) 0 (0%) 0 (0%) 0 (0%) 38 (54%) 18 (26%) 2 (3%) 0 (0%) 1 (1%) 1 (1%) 15 (68%) 9 (41%) 0 (0%) 0 (0%) 1 (5%) 0 (0%) Zeuzem S. Ann Intern Med 2015; 163:1-13 C-EDGE TN
C-EDGE TN Study : grazoprevir/elbasvir in genotype 1, 4 or 6 Laboratory abnormalities, n (%) GZR/EBR N = 316 Placebo N = 105 Late elevation of ALT or AST > 2.0-5.0 x ULN > 5.0 x ULN Elevation of total bilirubin > 2.5-5.0 x ULN > 5.0 x ULN Decreased hemoglobin Grade 1-2 Grade 3-4 Increased creatinine Grade 1 Grade 2 Increased lipase Grade 1-2 Grade 3-4 3 (1.0%) 4 (1.3%) 4 (3.8%) 0 (0%) 3 (0.9%) 1 (0.3%) 0 (0%) 0 (0%) 9 (2.9%) 0 (0%) 4 (3.8%) 0 (0%) 2 (0.6%) 0 (0%) 1 (1.0%) 0 (0%) 101 (32.0%) 19 (6.0%) 25 (23.8%) 5 (4.8%) Zeuzem S. Ann Intern Med 2015; 163:1-13 C-EDGE TN
C-EDGE TN Study: grazoprevir/elbasvir in genotype 1, 4 or 6 Summary A 12-week regimen of the oral fixed dose combination of once-daily, single-tablet of grazoprevir/elbasvir, achieved an overall SVR12of 95% High efficacy in genotypes 1 and 4 SVR12lower in genotype 6 High efficacy in cirrhotics (SVR12= 97.1%) Lower efficacy observed among patients with high viral load (HCV RNA > 800,000 IU/ml) Overall virologic failure rate was 4% Baseline NS3 RAVS did not affect efficacy Association between virologic failure and the presence of baseline NS5A RAVs, which was most apparent in genotype 1a with baseline RAVs demonstrating > 5-fold potency reduction to elbasvir Emergence of (additional) NS3 and/or NS5A RAVs at failure was common Grazoprevir/elbasvir was generally well-tolerated, with a similar safety profile in cirrhotic and non-cirrhotic patients Limitations No active-control group Zeuzem S. Ann Intern Med 2015; 163:1-13 C-EDGE TN