Genotype-Directed Dosing for Efavirenz in Children Study
The study focuses on genotype-directed dosing of Efavirenz in children with HIV, aiming to optimize treatment options and dosages based on genetic factors. It explores the challenges in treating children with HIV, especially in resource-limited settings, and discusses the impact of genetic variations on Efavirenz metabolism. The presentation outlines the global HIV burden in children, treatment options, and the P1070 study's dose-finding and pharmacogenetic aspects. It highlights the importance of personalized medicine in pediatric HIV care to enhance treatment efficacy and safety.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Genotype-directed dosing for Efavirenz T. G MBENGERANWA Harare Family Care CRS IMPAACT, International Maternal Pediatric Adolescent AIDS Clinical Trials Group
Presentation Outline Global HIV Burden in Children Treatment Options in Children IMPAACT P1070 Study IMPAACT, International Maternal Pediatric Adolescent AIDS Clinical Trials Group 2
Global Picture of HIV in children IMPAACT, International Maternal Pediatric Adolescent AIDS Clinical Trials Group 3
Treatment of HIV in children Treatment for children works!!! Options for children lag behind significantly (drugs and formulations) It can be complicated requiring pills and liquids Difficult to swallow Unpleasant Some medicines have temperature restrictions Electrical outages Storage facilities IMPAACT, International Maternal Pediatric Adolescent AIDS Clinical Trials Group 4
ART options for children in Zimbabwe IMPAACT, International Maternal Pediatric Adolescent AIDS Clinical Trials Group 5
P1070 STUDY Dose Finding and Pharmacogenetic study of Efavirenz (EFV) in HIV- Infected and HIV/TB Co- Infected Infants & Children < 36 months of age IMPAACT, International Maternal Pediatric Adolescent AIDS Clinical Trials Group
Background (1) Recommended as 1st line treatment for adults and children >3yr/>10kg body weight EFV metabolism Metabolized through the CYP 2B6 enzyme system Significant pharmacokinetic (PK) variability of EFV is related to a single polymorphism in the cytochrome (CYP) P450 2B6 gene Prevalence of the CYP 2B6 516TT genotype ranges 9-23% 516 TT are poor metabolizers slower clearance 516 GG and 516 GT are extensive metabolizers rapid clearance IMPAACT, International Maternal Pediatric Adolescent AIDS Clinical Trials Group 7
Background (2) Dose not established in children <3yr or <10kg body weight EFV is an NNRTI with a relatively narrow therapeutic window and serum concentrations showing high inter- and intra-patient variability Liquid formulations have shown erratic absorption and high variability P1070: Capsules opened into porridge, formula or expressed breastmilk IMPAACT, International Maternal Pediatric Adolescent AIDS Clinical Trials Group 8
Primary Objectives Determine dose of EFV administered as opened capsules to HIV-infected OR HIV/TB co-infected infants (taking anti-TB therapy [ATT]) between 3-<36 months of age 24 week safety and tolerance of EFV Explore the influence of genetic polymorphisms on EFV levels Evaluate CNS toxicity and association with EFV PK levels IMPAACT, International Maternal Pediatric Adolescent AIDS Clinical Trials Group 9
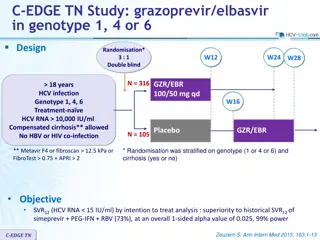
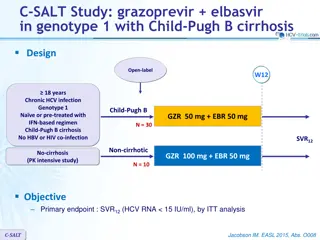
Study Design Stratification/Sample Size IMPAACT, International Maternal Pediatric Adolescent AIDS Clinical Trials Group 10
EFV Dosing Approach Starting EFV dose was modelled using data from previous EFV trials (P382 and P1021) Version 1.0 of the study used weight band dosing (approx. 40mg/kg/day) targeting AUC target of 35- 180 mcg*hr/L Real-time CYP 2B6 516 was assessed IMPAACT, International Maternal Pediatric Adolescent AIDS Clinical Trials Group 11
Baseline Characteristics by CYP 2B6 516 genotype CYP 2B6 516 genotype Characteristic 516 GG/GT (N=38) 516 TT (N=9) Total (N=47) Age (mo) median 19 20 19 Gender M 24 (63%) 4 (44%) 28 (60%) F 14 (37%) 5 (56%) 19 (40%) Race Asian 9(24%) 2 (22%) 11 (23%) Black 28 (74%) 7 (78%) 35 (74%) Unknown 1 (3%) 0 (0%) 1 (2%) CYP 2B6 516 genotype GG 21 (55%) 0 (0%) 21(45%) GT 17(45%) 0 (0%) 17 (36%) TT 0(0%) 9 (100%) 9 (19%) IMPAACT, International Maternal Pediatric Adolescent AIDS Clinical Trials Group 12
Baseline Characteristics by CYP 2B6 516 genotype CYP 2B6 516 genotype Characteristic 516 GG/GT (N=38) 516 TT (N=9) Total (N=47) Log10 baseline RNA Median 5.79 5.83 5.8 Q1,Q3 13.27 18.26 13.27 Baseline CD4 % Median 20.6 17.9 19 Q1,Q3 15, 27.1 12,21 14,26 IMPAACT, International Maternal Pediatric Adolescent AIDS Clinical Trials Group 13
Week 2 Intensive (24 hour) PK Prior to observed dose and 2, 4, 8, 12 and 24 hours post dose DBS samples (CYP 2B6 516) shipped real time Results of intensive PK were used to determine the need for individual dose adjustment IMPAACT, International Maternal Pediatric Adolescent AIDS Clinical Trials Group 14
Week 2 PK results IMPAACT, International Maternal Pediatric Adolescent AIDS Clinical Trials Group 15
24 Hour Plasma PK at Week 2 EFV Cohort 1 (SEP 11) 20 Median EFV Plasma Conc (mcg/mL) P1070 Dose GG/ GT 16 12 P1070v1 Dose TT 8 4 0 P1070v2 Dose TT 0 4 8 12 16 20 24 Time After Dose IMPAACT, International Maternal Pediatric Adolescent AIDS Clinical Trials Group Initially median EFV AUC was higher in TT genotype vs GG/GT (median 490 vs 107 p=0.0001) 16
Percentage of AUCs in Target Range 100 Percentage of EFV AUCs in Target 80 60 40 20 0 GG/ GT - v1&2 GG/ GT - FDA TT - v1 TT - v2 TT - FDA CYP2B6 Genotype Group / Version Dose In Target Below Above Modelling using P1070 data predicted that FDA-approved doses would produce sub-therapeutic AUCs in almost one third of participants with 516GG/GT and excessive AUCs in >50% with 516TT genotypes IMPAACT, International Maternal Pediatric Adolescent AIDS Clinical Trials Group 17
Safety and Virology EFV was well tolerated No disproportionate CNS toxicity in children with excessive EFV levels 15/47 experienced toxicities deemed as possibly treatment related (11 GG/GT, 4 TT) Week 24 virologic suppression (< 400 copies/ml): 73% IMPAACT, International Maternal Pediatric Adolescent AIDS Clinical Trials Group 18
Summary of Cohort I Results AUC GG/GT AUC TT Safety Virologic suppression IMPAACT, International Maternal Pediatric Adolescent AIDS Clinical Trials Group 19
Conclusion EFV remains an important treatment option in RLS Significant inter participant PK variations due to the CYP2B6 genotype challenge the use of uniform EFV dosages for <3 years of age Genotype- directed EFV dosing can mitigate this challenge These tests can be processed in Africa however they are still costly Use of EFV in <3yrs increases the treatment options for children living with HIV IMPAACT, International Maternal Pediatric Adolescent AIDS Clinical Trials Group 20
Acknowledgements IMPAACT, International Maternal Pediatric Adolescent AIDS Clinical Trials Group 21