C-EDGE.CO-STAR: Grazoprevir/Elbasvir for HCV Infected Drug Users on Opioid Replacement Therapy
A study on the effectiveness of grazoprevir/elbasvir in treating HCV-infected individuals on opioid replacement therapy. The study aimed to achieve SVR12 with immediate treatment and compared results to deferred treatment. The primary endpoint SVR12 was achieved in a high percentage of patients on immediate treatment, demonstrating the superiority of immediate treatment over deferred treatment.
Uploaded on Nov 18, 2024 | 0 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
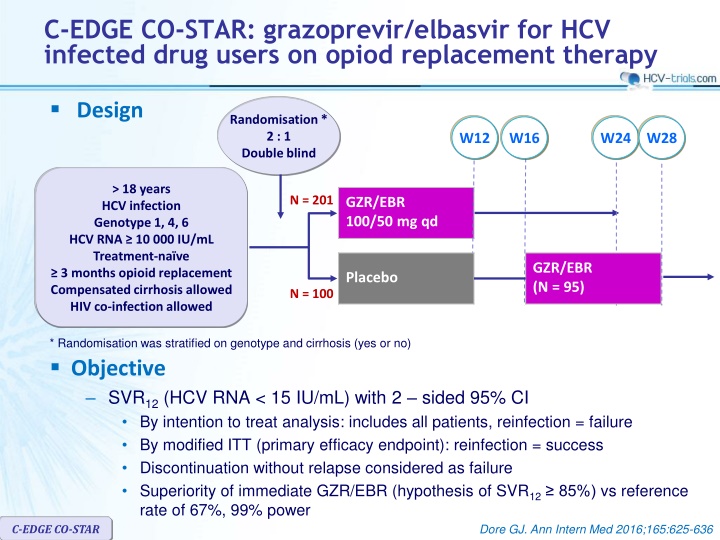
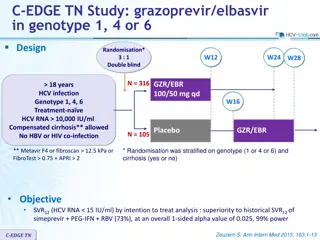
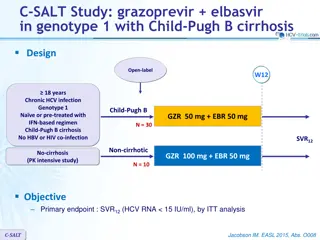
C-EDGE CO-STAR: grazoprevir/elbasvir for HCV infected drug users on opiod replacement therapy Design Randomisation * 2 : 1 Double blind W12 W16 W24 W28 > 18 years HCV infection Genotype 1, 4, 6 HCV RNA 10 000 IU/mL Treatment-na ve 3 months opioid replacement Compensated cirrhosis allowed HIV co-infection allowed N = 201 GZR/EBR 100/50 mg qd GZR/EBR (N = 95) Placebo N = 100 * Randomisation was stratified on genotype and cirrhosis (yes or no) Objective SVR12(HCV RNA < 15 IU/mL) with 2 sided 95% CI By intention to treat analysis: includes all patients, reinfection = failure By modified ITT (primary efficacy endpoint): reinfection = success Discontinuation without relapse considered as failure Superiority of immediate GZR/EBR (hypothesis of SVR12 85%) vs reference rate of 67%, 99% power C-EDGE CO-STAR Dore GJ. Ann Intern Med 2016;165:625-636
C-EDGE CO-STAR: grazoprevir/elbasvir for HCV infected drug users on opiod replacement therapy Baseline characteristics and patient disposition GZR/EBR N = 201 48 24 15 / 78 / 5 Placebo N = 100 47 23 7 / 84 / 7 Age, years, median Female, % Black / White / Asian, % Genotype, % 1a 1b 4 6 IL28B CC, % HCV RNA > 2 000 000 IU/mL, % Fibrosis stage F4, % HIV co-infection, % Urine drug screen positive at D1, % Discontinuation before W12 of follow-up (active phase), N Lost to follow-up For adverse event Administrative reason 76 15 6 2.5 28.4 56.7 19.9 8.0 62.2 75 15 6 4 29.0 51.0 22.0 5.0 53.1 5 3 1 1 7 7 0 0 C-EDGE CO-STAR Dore GJ. Ann Intern Med 2016;165:625-636
C-EDGE CO-STAR: grazoprevir/elbasvir for HCV infected drug users on opiod replacement therapy Primary endpoint: SVR12(HCV RNA < 15 IU/mL), % (95% CI), ITT mFAS* Immediate GZR/EBR 95.5 % 94.0 93.3 91.7 (90.9-98.2) 100 (89.8-96.0) (77.9-99.2) (61.5-99.8) 75 60 (14.7-94.7) 50 25 201 154 30 12 5 N= 0 All patients Genotype 1a Genotype 1b Genotype 4 Genotype 6 SVR12, ITT, Full analysis set 91.5% 93.5% 93.3% 91.7% 20% Non-virologic failure Relapse Reinfection 5 7 5 3 4 3 1 1 0 1 0 0 0 2 2 * ITT, mFAS: reinfection = success, unrelated discontinuation = excluded C-EDGE CO-STAR Dore GJ. Ann Intern Med 2016;165:625-636
C-EDGE CO-STAR: grazoprevir/elbasvir for HCV infected drug users on opiod replacement therapy Primary endpoint: SVR12(HCV RNA < 15 IU/mL), % (95% CI), ITT mFAS* Deferred GZR/EBR 100 % 92.9 90.1 (54.1-100) 89.5 100 (66.1-99.8) (80.7-95.9) (81.5-94.8) 75 50 (6.8-93.2) 50 25 95 71 14 6 4 N= 0 All patients Genotype 1a Genotype 1b Genotype 4 Genotype 6 SVR12, ITT, Full analysis set 89.5% 90.1% 92.9% 100% 50% Non-virologic failure Relapse Breakthrough Reinfection 7 1 2 0 6 0 1 0 1 0 0 0 0 0 0 0 0 1 1 0 * ITT, mFAS: reinfection = success, unrelated discontinuation = excluded C-EDGE CO-STAR Dore GJ. Ann Intern Med 2016;165:625-636
C-EDGE CO-STAR: grazoprevir/elbasvir for HCV infected drug users on opiod replacement therapy SVR12(HCV RNA < 15 IU/mL) in the immediate treatment group, by subgroup, % (95% CI), ITT, Full analysis set * % 93.8 92.8 92.5 92.0 90.4 91.3 91.2 87.5 100 (85-98.3) (87.5-96.4) (79.6-98.4) (84.3-96.7) (84.2-94.8) (85.8-95.2) (84.3-95.7) (74.8-95.3) 75 50 25 153 48 136 65 161 40 88 113 0 Male Female Positive Negative No Yes No Yes Sex Drug screen Cirrhosis HCV RNA > 2 000 000 IU/mL * Reinfection = failure C-EDGE CO-STAR Dore GJ. Ann Intern Med 2016;165:625-636
C-EDGE CO-STAR: grazoprevir/elbasvir for HCV infected drug users on opiod replacement therapy SVR12and SVR24(HCV RNA < 15 IU/mL), %, per protocol Immediate treatment Deferred treatment % 100 96.6 96.5 95.5 94.1 75 50 25 N= 198 186 88 85 0 SVR12 SVR24 SVR12 SVR24 C-EDGE CO-STAR Dore GJ. Ann Intern Med 2016;165:625-636
C-EDGE CO-STAR: grazoprevir/elbasvir for HCV infected drug users on opiod replacement therapy Urine drug screen results, from D1 to treatment W12 At each visit, in both groups > 50% of patients with positive urine drug screen of any drug among the 8 following classes: amphetamines, barbiturates, benzodiazepines, cannabinoids, cocaine, opiates, phencyclidine, propoxyphene Positive UDS results at baseline or during treatment (2 or more positive UDS results) did not affect adherence or efficacy, regardless of which drug class was positive on UDS Adherence to study drugs Deferred GZR/EBR Immediate GZR/EBR Placebo phase Active phase Adherence > 95% during the 12 weeks 96.5% 100% 95.8% C-EDGE CO-STAR Dore GJ. Ann Intern Med 2016;165:625-636
C-EDGE CO-STAR: grazoprevir/elbasvir for HCV infected drug users on opiod replacement therapy SVR12and urine drug screen results in the immediate treatment group (all randomised patients) SVR12 Subgroup n/N % (95% CI) Opiod agonist therapy at D1 Buprenorphine Methadone 37/39 147/162 94.9 (82.7-99.4) 90.7 (85.2-94.7) Drug screen during the treatment period Positive (at least 2 time points) Negative 123/136 61/65 90.4 (84.2-94.8) 93.8 (85.0-98.3) C-EDGE CO-STAR Dore GJ. Ann Intern Med 2016;165:625-636
C-EDGE CO-STAR: grazoprevir/elbasvir for HCV infected drug users on opiod replacement therapy Reinfection % Reinfections Virologic failures 3.0 2.0 1.0 0.0 FW8 TW10 or 12 FW4 FW12 FW24 Reinfection, N (%) Virologic failure, N (%) 0 (0.0) 2 (0.7) 0 (0.0) 3 (1.0) 5 (1.7) 3 (1.0) 0 (0.0) 2 (0.7) 1 (0.3) 2 (0.7) Incidence of reinfection 4.6 reinfections (CI, 1.7 to 10.0) per 100 person-years 6 reinfections: different genotype, subtype or viral strain Dore G. EASL 2016, Abs. SAT-163, J Hepatol 2016;64:S771 ; Dore GJ. Ann Intern Med 2016;165:625-636 C-EDGE CO-STAR
C-EDGE CO-STAR: grazoprevir/elbasvir for HCV infected drug users on opiod replacement therapy Summary of 18 patients with virological failure or probable reinfection Age, sex, cirrhotic status HCV RNA Genotype Drug screen Type/Time of failure 61-y, white male, cirrhosis 5.6 million IU/mL 1a Positive Relapse follow-up W12 50-y, black male, cirrhosis 13.6 million IU/mL 1a Negative Relapse follow-up W4 56-y, male, no cirrhosis 1.2 million IU/mL 1a Positive Relapse follow-up W8 63-y, white female, no cirrhosis 3.5 million IU/mL 1a Positive Relapse follow-up W4 63-y, black female, no cirrhosis 1.7 million IU/mL 1b Negative Relapse follow-up W12 29-y, asian male, no cirhhosis 33.2 million IU/mL 6a Positive Relapse follow-up W4 34-y, asian male, no cirrhosis 25.5 million IU/mL 6a Positive Relapse follow-up W8 29-y, white female, no cirrhosis 335 157 IU/mL 1a Not done Relapse follow-up W24 50-y, black female, cirrhosis 9.5 million IU/mL 1b Positive Relapse follow-up W24 58-y, black male, cirrhosis 2.8 million IU/mL 1a Positive Breakthrough W12 59-y, asian male, no cirrhosis 1.9 million IU/mL 6b Negative Relapse follow-up W8 43-y, asian male, no cirrhosis 6.8 million IU/mL 6a Positive Breakthrough W12 48-y, asian male, no cirrhosis 17 274 IU/mL 1a Positive Reinfection (GT 6a) FU W8 33-y, white female, no cirrhosis 535 293 IU/mL 1a Positive Reinfection (GT 1a) FU W8 55-y, white female, cirrhosis 3.2 million IU/mL 1a Positive Reinfection (GT 3a) FU W8 45-y, asian male, no cirrhosis 4.8 million IU/mL 6a Positive Reinfection (GT 1b) FU W8 37-y, asian female, no cirrhosis 18.6 million IU/mL 6a Positive Reinfection (GT 6a) FU W8 33-y, white male, no cirrhosis 1.5 million IU/mL 1b Not done Reinfection (GT 3a) FU W24 C-EDGE CO-STAR Dore GJ. Ann Intern Med 2016;165:625-636
C-EDGE CO-STAR: grazoprevir/elbasvir for HCV infected drug users on opiod replacement therapy Reinfection during post-treatment and follow-up (3 years post-treatment) Reinfection rate per 100 person- years (95% CI) Reinfections N Follow-up person-years All persons 10 565 1.8 (0.8 3.3) Post-treatment to 3 years-follow-up Persons who reported IDU Persons who did not report IDU 6 1 212 316 2.8 (1.0 6.2) 0.3 (0.0 1.8) IDU = intravenous drug use C-EDGE CO-STAR Dore GJ. Ann Intern Med 2016;165:625-636 ; Grebely J. AASLD 2018 ; Abs. 52
C-EDGE CO-STAR: grazoprevir/elbasvir for HCV infected drug users on opiod replacement therapy Reinfection % Reinfections Virologic failures 3.0 2.0 1.0 0.0 FW8 TW10 or 12 FW4 FW12 FW24 Reinfection, N (%) Virologic failure, N (%) 0 (0.0) 2 (0.7) 0 (0.0) 3 (1.0) 5 (1.7) 3 (1.0) 0 (0.0) 2 (0.7) 1 (0.3) 2 (0.7) Incidence of reinfection 4.6 reinfections (CI, 1.7 to 10.0) per 100 person-years 6 reinfections: different genotype, subtype or viral strain Dore G. EASL 2016, Abs. SAT-163, J Hepatol 2016;64:S771 ; Dore GJ. Ann Intern Med 2016;165:625-636 C-EDGE CO-STAR
C-EDGE CO-STAR: grazoprevir/elbasvir for HCV infected drug users on opiod replacement therapy Adverse events, % Deferred GZR/EBR GZR/EBR N = 201 Placebo phase N = 100 Active phase N = 95 Serious adverse event Serious drug-related adverse event 3.5 0.5 4.0 1.0 3.0 0 Adverse event leading to discontinuation 0.5 1.0 0 Most frequent adverse events Fatigue Headache Nausea AST/ALT > 3 x ULN Total bilirubin > 2.6 x ULN Hemoglobin < 8.5 g/dL Creatinine > 2.5 times baseline level 15.9 12.4 10.9 0 0 0.5 0 20.0 13.0 9.0 2 0 1 0 13.7 12.6 7.4 0 0 1.1 0 C-EDGE CO-STAR Dore GJ. Ann Intern Med 2016;165:625-636
C-EDGE CO-STAR: grazoprevir/elbasvir for HCV infected drug users on opiod replacement therapy Summary EBR/GZR demonstrated high efficacy in genotype 1 and 4-infected patients receiving opiate agonist therapy Limitation: small number of genotype 6-infected patients Acceptable safety profile with comparable adverse event rates between the immediate and deferred treatment arms High study medication adherence Stable ongoing drug use throughout the initial treatment phase in both groups Reinfection rate is higher in the immediate follow-up period These results support the removal of drug use as a barrier to interferon- free HCV treatment for patients receiving opiate agonist therapy C-EDGE CO-STAR Dore GJ. Ann Intern Med 2016;165:625-636
C-EDGE CO-STAR: grazoprevir/elbasvir for HCV infected drug users on opiod replacement therapy 3-year Follow-up on Risk Factors and Rate of Reinfection Observational cohort (6-month follow-up visits) Follow-up of 199/296 (67%) patients enrolled in Co-STAR Positive urine drug screen (UDS) at enrollment: 56% and 58% of patients in the 3YFU study and the Phase 3 trial, respectively Median time from EOT to the first visit during the 3YFU was 330 days (range: 206-485) 84 (56%) patients reported any drug use (non-injecting or injecting) in the past 6 months. Injecting drug use in the past 6 months was reported by 25% of patients Reinfection rate : higher in the immediate follow-up period through week (FW)12 (N = 5) than in the period through FW24 (+1) and through the ongoing observational follow-up Overall reinfection rate through the 6-month follow-up period is 4.0/100 person- years (N = 8) [ 95% CI : 1.7-8.0] Including only those patients with persistence of viremia (N = 5), the effective reinfection rate is 2.5/100 person-years [95% CI : 0.8-5.9] These data support addressing barriers in the treatment of patients on opiate agonist therapy and patients with ongoing drug use C-EDGE CO-STAR Dore GJ. AASLD 2016, Abs. 871