Understanding Alkenes, Alcohols, and Carboxylic Acids in Organic Chemistry
Alkenes are hydrocarbons with a double carbon-carbon bond, reacting uniquely due to their functional group. Alcohols and carboxylic acids also have distinctive properties and reactions, important in organic chemistry studies. Learn about their structures, formulas, reactions with other compounds, and their significance in chemical processes.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
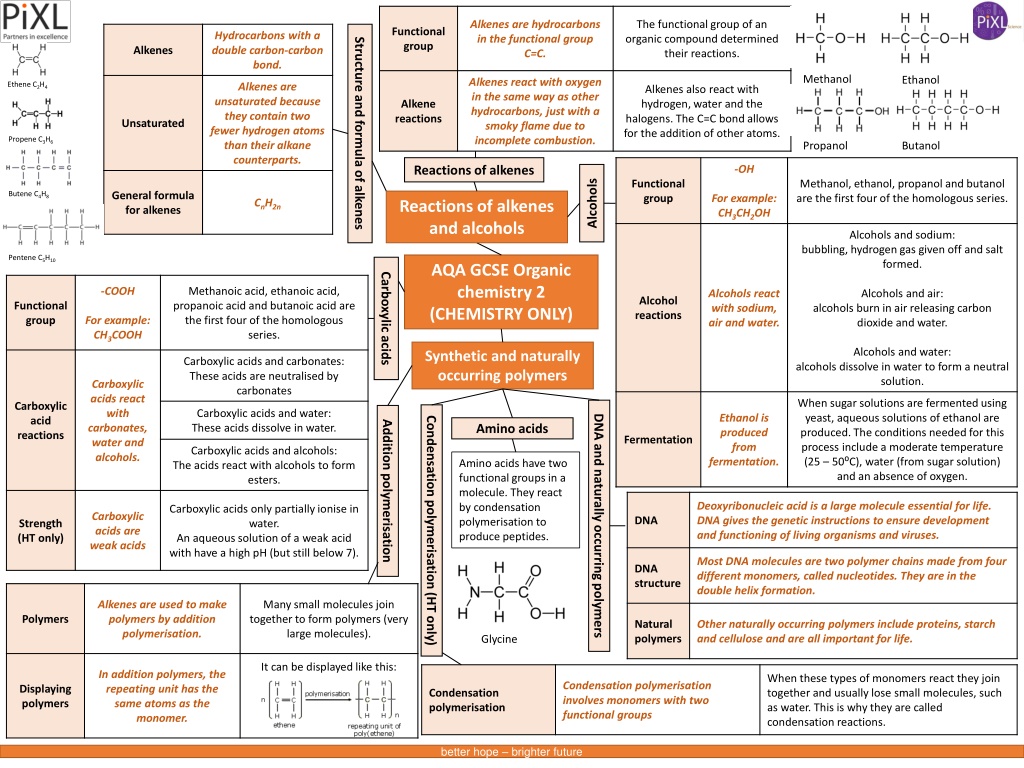
Alkenes are hydrocarbons in the functional group C=C. The functional group of an organic compound determined their reactions. Functional group Hydrocarbons with a double carbon-carbon bond. Structure and formula of alkenes Alkenes Methanol Ethanol Alkenes react with oxygen in the same way as other hydrocarbons, just with a smoky flame due to incomplete combustion. Ethene C2H4 Alkenes are unsaturated because they contain two fewer hydrogen atoms than their alkane counterparts. Alkenes also react with hydrogen, water and the halogens. The C=C bond allows for the addition of other atoms. Alkene reactions Unsaturated Propene C3H6 Butanol Propanol -OH Reactions of alkenes Alcohols Functional group Methanol, ethanol, propanol and butanol are the first four of the homologous series. General formula for alkenes Butene C4H8 For example: CH3CH2OH CnH2n Reactions of alkenes and alcohols Alcohols and sodium: bubbling, hydrogen gas given off and salt formed. Pentene C5H10 AQA GCSE Organic chemistry 2 (CHEMISTRY ONLY) Carboxylic acids -COOH Methanoic acid, ethanoic acid, propanoic acid and butanoic acid are the first four of the homologous series. Alcohols react with sodium, air and water. Alcohols and air: Alcohol reactions Functional group alcohols burn in air releasing carbon dioxide and water. For example: CH3COOH Alcohols and water: Synthetic and naturally occurring polymers Carboxylic acids and carbonates: These acids are neutralised by carbonates alcohols dissolve in water to form a neutral solution. Carboxylic acids react with carbonates, water and alcohols. When sugar solutions are fermented using yeast, aqueous solutions of ethanol are produced. The conditions needed for this process include a moderate temperature (25 50 C), water (from sugar solution) and an absence of oxygen. Carboxylic acid reactions Carboxylic acids and water: These acids dissolve in water. Ethanol is produced from fermentation. DNA and naturally occurring polymers Condensation polymerisation (HT only) Addition polymerisation Amino acids Fermentation Carboxylic acids and alcohols: The acids react with alcohols to form esters. Amino acids have two functional groups in a molecule. They react by condensation polymerisation to produce peptides. Deoxyribonucleic acid is a large molecule essential for life. DNA gives the genetic instructions to ensure development and functioning of living organisms and viruses. Carboxylic acids only partially ionise in water. An aqueous solution of a weak acid with have a high pH (but still below 7). Carboxylic acids are weak acids DNA Strength (HT only) Most DNA molecules are two polymer chains made from four different monomers, called nucleotides. They are in the double helix formation. DNA structure Alkenes are used to make polymers by addition polymerisation. Many small molecules join together to form polymers (very large molecules). Polymers Natural polymers Other naturally occurring polymers include proteins, starch and cellulose and are all important for life. Glycine It can be displayed like this: In addition polymers, the repeating unit has the same atoms as the monomer. When these types of monomers react they join together and usually lose small molecules, such as water. This is why they are called condensation reactions. Condensation polymerisation involves monomers with two functional groups Displaying polymers Condensation polymerisation better hope brighter future
Alkenes are hydrocarbons in the functional group C=C. The functional group of an organic compound determined their reactions. Hydrocarbons with a double carbon-carbon bond. Structure and formula of alkenes Methanol Ethanol Alkenes react with oxygen in the same way as other hydrocarbons, just with a smoky flame due to incomplete combustion. Ethene C2H4 Alkenes are unsaturated because they contain two fewer hydrogen atoms than their alkane counterparts. Alkenes also react with hydrogen, water and the halogens. The C=C bond allows for the addition of other atoms. Propene C3H6 Butanol Propanol -OH Reactions of alkenes Alcohols Methanol, ethanol, propanol and butanol are the first four of the homologous series. Butene C4H8 For example: CH3CH2OH CnH2n Reactions of alkenes and alcohols Alcohols and sodium: bubbling, hydrogen gas given off and salt formed. Pentene C5H10 AQA GCSE Organic chemistry 2 (CHEMISTRY ONLY) Carboxylic acids -COOH Methanoic acid, ethanoic acid, propanoic acid and butanoic acid are the first four of the homologous series. Alcohols react with sodium, air and water. Alcohols and air: alcohols burn in air releasing carbon dioxide and water. For example: CH3COOH Alcohols and water: Synthetic and naturally occurring polymers Carboxylic acids and carbonates: These acids are neutralised by carbonates alcohols dissolve in water to form a neutral solution. Carboxylic acids react with carbonates, water and alcohols. When sugar solutions are fermented using yeast, aqueous solutions of ethanol are produced. The conditions needed for this process include a moderate temperature (25 50 C), water (from sugar solution) and an absence of oxygen. Carboxylic acids and water: These acids dissolve in water. Ethanol is produced from fermentation. DNA and naturally occurring polymers Condensation polymerisation (HT only) Addition polymerisation Amino acids Carboxylic acids and alcohols: The acids react with alcohols to form esters. Amino acids have two functional groups in a molecule. They react by condensation polymerisation to produce peptides. Deoxyribonucleic acid is a large molecule essential for life. DNA gives the genetic instructions to ensure development and functioning of living organisms and viruses. Carboxylic acids only partially ionise in water. An aqueous solution of a weak acid with have a high pH (but still below 7). Carboxylic acids are weak acids Most DNA molecules are two polymer chains made from four different monomers, called nucleotides. They are in the double helix formation. Alkenes are used to make polymers by addition polymerisation. Many small molecules join together to form polymers (very large molecules). Other naturally occurring polymers include proteins, starch and cellulose and are all important for life. Glycine It can be displayed like this: In addition polymers, the repeating unit has the same atoms as the monomer. When these types of monomers react they join together and usually lose small molecules, such as water. This is why they are called condensation reactions. Condensation polymerisation involves monomers with two functional groups better hope brighter future
The functional group of an organic compound determined their reactions. Functional group Structure and formula of alkenes Alkenes Methanol Ethanol Ethene C2H4 Alkenes also react with hydrogen, water and the halogens. The C=C bond allows for the addition of other atoms. Alkene reactions Unsaturated Propene C3H6 Butanol Propanol Reactions of alkenes Functional group Methanol, ethanol, propanol and butanol are the first four of the homologous series. Alcohols Butene C4H8 Reactions of alkenes and alcohols General formula for alkenes Alcohols and sodium: bubbling, hydrogen gas given off and salt formed. Pentene C5H10 AQA GCSE Organic chemistry 2 (CHEMISTRY ONLY) Carboxylic acids Methanoic acid, ethanoic acid, propanoic acid and butanoic acid are the first four of the homologous series. Alcohols and air: Alcohol reactions Functional group alcohols burn in air releasing carbon dioxide and water. Alcohols and water: Synthetic and naturally occurring polymers Carboxylic acids and carbonates: These acids are neutralised by carbonates alcohols dissolve in water to form a neutral solution. When sugar solutions are fermented using yeast, aqueous solutions of ethanol are produced. The conditions needed for this process include a moderate temperature (25 50 C), water (from sugar solution) and an absence of oxygen. Carboxylic acid reactions Carboxylic acids and water: These acids dissolve in water. DNA and naturally occurring polymers Condensation polymerisation (HT only) Addition polymerisation Amino acids Fermentation Carboxylic acids and alcohols: The acids react with alcohols to form esters. Amino acids have two functional groups in a molecule. They react by condensation polymerisation to produce peptides. Carboxylic acids only partially ionise in water. An aqueous solution of a weak acid with have a high pH (but still below 7). DNA Strength (HT only) DNA structure Many small molecules join together to form polymers (very large molecules). Polymers Natural polymers Glycine It can be displayed like this: When these types of monomers react they join together and usually lose small molecules, such as water. This is why they are called condensation reactions. Displaying polymers Condensation polymerisation better hope brighter future
Functional group Structure and formula of alkenes Alkenes Methanol Ethanol Ethene C2H4 Alkene reactions Unsaturated Propene C3H6 Butanol Propanol Reactions of alkenes Alcohols Functional group Butene C4H8 Reactions of alkenes and alcohols General formula for alkenes Pentene C5H10 AQA GCSE Organic chemistry 2 (CHEMISTRY ONLY) Carboxylic acids Functional group Alcohol reactions Synthetic and naturally occurring polymers Carboxylic acid reactions DNA and naturally occurring polymers Condensation polymerisation (HT only) Addition polymerisation Amino acids Fermentation Amino acids have two functional groups in a molecule. They react by condensation polymerisation to produce peptides. Strength (HT only) DNA DNA structure Polymers Natural polymers Glycine It can be displayed like this: Displaying polymers Condensation polymerisation better hope brighter future