Understanding Ionization of Carboxylic Acids
Carboxylic acids, as proton donors, can undergo ionization to form ions in chemical reactions based on the Brønsted-Lowry theory. Ionization involves the complete loss or gain of electrons, leading to the formation of cations (positively charged ions) and anions (negatively charged ions). Through examples and explanations, the process of ionization of carboxylic acids is elucidated, highlighting the significance of this chemical phenomenon in understanding acid-base interactions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
IONIZATION OF CARBOXYLIC ACIDS
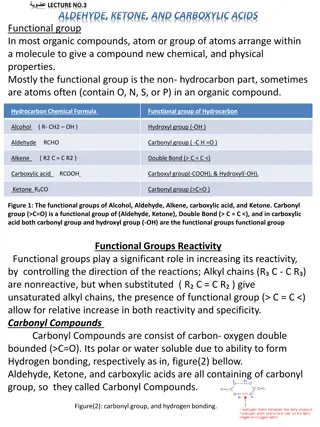
CARBOXYLIC ACIDS An acid is a molecule or ion capable of donating a proton, or, alternatively, capable of forming a covalent bond with an electron pair. The first category of acids are the proton donors, or Br nsted Lowry acids.
BrnstedLowry theory, also called proton theory of acids and bases, a theory, introduced independently in 1923 by the Danish chemist Br nsted and the English chemist Thomas Martin Lowry, stating that any compound that can transfer a proton to any other compound is an acid, and the compound that accepts the proton is a base. A proton is a nuclear particle with a unit positive electrical charge; it is represented by the it constitutes the nucleus of a hydrogen atom. Johannes Nicolaus symbol H+ because
What is IONIZATION Strictly defined, ionization is the complete loss of an electron from an atomic or molecular species. The resulting species is called an ion. In chemical equations, the charge on ions is shown as a superscript, such as in this simple ionization reaction: M M++ e- Ions may ionize further: M+ M2++ e- M2+ M3++ e- M3+ ..... etc
Cations Positively charged ions are often referred to as cations. Negative Ions / Anions Although in strict terms ionization refers to the formation of a positive ion, in normal usage, the word also includes the formation of a negative ion: M + e- M- Negatively charged ions are often referred to as anions.
Ionization Examples Direct Ionization of Elements Metals typically form cations and non-metals typically form anions. Some elements, such as carbon, gold, and the noble gases, do not readily form ions. The alkali metals in Group 1 of the periodic table and the halides in Group 17 ionize very readily. Alkali metals need only lose one electron to obtain a full electron shell: likewise, halides need only gain one electron to achieve this. For example, sodium and chlorine react spontaneously by ionizing to form the ionic compound sodium chloride: 2Na(s) + Cl2(g) 2NaCl(s)
Define ionization of carboxilyic acid : Water-soluble carboxylic acids ionize slightly in water to form moderately acidic solutions. Their aqueous solutions exhibit the typical properties of acids, such as changing litmus from blue to red. The anion formed when acid dissociates is called the carboxylate anion (RCOO ). carboxylic a
Acid Strength and the Acid Dissociation Constant (Ka) 1. The magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids and bases. For example, the general equation for the ionization of a weak acid in water, where HA is the parent acid and A is its conjugate base, is as follows:
HA(aq)+H2O(l)H3O+(aq)+A(aq) The equilibrium constant for this dissociation is as follows: K=[H3O+][A ][H2O][HA]
As we noted earlier, the concentration of water is essentially constant for all reactions in aqueous solution, so Equation 16.4.216.4.2 can be incorporated into a new quantity, the acid ionization constant (KaKa), also called the acid dissociation constant: Ka=K[H2O]=[H3O+][A ][HA](16.4.3)(16.4.3) Ka=K[H2O]=[H3O+][A ][HA] [H2O][H2O] in
Thus the numerical values of K and KaKa differ by the concentration of water simplicity, H3O+H3O+ can be written as H+H+ in Equation 16.4.316.4.3. Keep in mind, though, that free H+H+ does not exist in aqueous solutions and that a proton is transferred to H2OH2O in all acid ionization reactions to form hydronium ions, H3O+H3O+. The larger the KaKa, the stronger the acid and the higher the H+H+ concentration at equilibrium. Like all equilibrium constants, acid base ionization constants are actually measured in terms of the activities of H+H+ or OH OH , thus making them unitless. The values of KaKa for a number of common acids are given in Table (55.3 M). Again, for
Table 16.4.116.4.1: Values of KaKa, pKapKa, KbKb, and pKbpKb for Selected Acids (HAHA and Their Conjugate Bases (A A ) Acid HAHA KaKa *The number in parentheses indicates the ionization step referred to for a polyprotic acid. 2 1092 1 09 H2SO4H2S O4 02 HNO3HNO 3 3 101 pKapKa A A KbKb pKbpKb 5.5 10 24 5.5 10 24 1 10 161 10 16 4.3 10 16 4.3 10 16 hydroiodic acid HIHI 9.3 I I 23.26 1 1021 1 HSO 4HSO 4 NO 3NO3 sulfuric acid (1)* 2.0 16.0 2.3 1012. nitric acid 1.37 15.37 H3O+H3O + H2OH2O1.0 10 14 1.0 10 14 hydronium ion 1.01.0 0.00 14.00 HSO 4HSO 4 1.0 10 21 .0 10 2 SO2 4SO4 2 9.8 10 13 9.8 10 13 sulfuric acid (2)* 1.99 12.01 6.3 10 46 .3 10 4 1.6 10 11 1.6 10 11 hydrofluoric acid HFHF 3.20 F F 10.80 HNO2HNO 2 5.6 10 45 .6 10 4 NO2 NO2 1.8 10 11 1.8 10 11 nitrous acid 3.25 10.75