Understanding Voltammetry: Principles and Applications
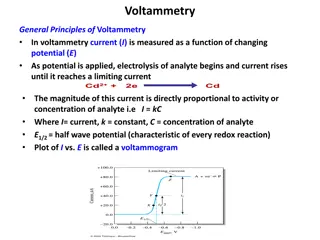
In voltammetry, current is measured as a function of changing potential. The magnitude of current is directly proportional to the activity or concentration of the analyte. A voltammogram is plotted between current and potential, showing the characteristic half-wave potential. The process involves a
6 views • 76 slides
Understanding Solutions and Precipitation Reactions
Solutions are classified as saturated, unsaturated, or supersaturated based on the concentration of solute in the solvent. Solubility plays a critical role in determining the amount of solute that can be dissolved in a solvent at a given temperature. Reactions yielding products of limited solubility
6 views • 10 slides
Chemical Reactions: Vocabulary Practice and Experiments in Chemistry 101
In this additional text for practicing chemical reactions vocabulary, students in Chemistry 101 conduct an experiment by combining a solvent and a solute to observe a simple chemical reaction, focusing on the proper process and product description. The materials, instructions, and objectives are pro
0 views • 8 slides
Understanding Nerves and Muscles Physiology: Lecture Insights by Dr. Amar AL-Musawi MD, PhD
Delve into the intricacies of cardiac action potential, mechanism of action potential propagation, rhythmicity in excitable tissues, and the crucial concept of refractory period. Explore how voltage-gated channels influence the duration of action potential and learn about the spontaneous generation
0 views • 12 slides
Understanding Water Potential and Solute Concentration in Biological Systems
Explore the concept of water potential and solute concentration in cells through diagrams and equations. Learn about hypertonic, hypotonic, and isotonic solutions, and how water movement is affected by solute concentration differences. Discover how adding solutes affects water potential and the dire
0 views • 19 slides
Understanding Solubility of Organic Compounds
A solution is a homogeneous mixture composed of a solute dissolved in a solvent. Solubility refers to the ability of one compound to dissolve in another. The solubility of organic compounds can be categorized based on chemical reactions like acid-base interactions. Different methods such as gravity
0 views • 6 slides
Gravitational Potential Energy Equations and Examples
Learn how to rearrange the potential energy equation, calculate potential energy using the formula PE = mgh, and solve for mass and height in relation to gravitational potential energy. Explore examples of calculating potential energy for objects at different heights. Understand the concept of gravi
0 views • 11 slides
Understanding Water Potential and Osmosis in Plants
This content delves into the concept of water potential, osmosis, and pressure potential in plant biology. It explains how water moves within plant cells, the unit of measurement for water potential, the role of osmotic potential in water movement, and the effects of solute concentration on water po
3 views • 18 slides
Understanding Standard Solutions in Chemistry
Definitions of terms like solute, solvent, and saturation are explained. The importance of preparing standard solutions, methods for their preparation, and how results are expressed are discussed. Stock solutions and their benefits are also covered. Images are used for illustration throughout the co
1 views • 65 slides
Understanding Concentration in Solutions
Solutions involve the dissolution of solutes like salt or sugar in solvents such as water, resulting in different concentrations. This concentration can be expressed as moles per liter, known as molarity. By calculating the amount of substance in moles using solution volume and concentration, you ca
0 views • 8 slides
Colligative Properties in Solutions: Vapor Pressure, Freezing Point Depression, and Osmotic Pressure
Colligative properties such as vapor pressure lowering, freezing point depression, and osmotic pressure are characteristics of solutions that depend on the number of solute particles present. This text explores how these properties are related to the concentration of solute in a solution and how the
0 views • 14 slides
Understanding Potentiometry: Principles, Methods, and Applications
Potentiometry is a field of electroanalytical chemistry that measures potential under no current flow conditions. It involves determining solute concentrations in solutions by measuring potential differences between electrodes. The history, principles, procedures, and applications of potentiometry a
0 views • 33 slides
Understanding Urolithiasis: Causes, Diagnosis, and Management
Urolithiasis, commonly known as kidney stones, has evolved over the years with changes in patterns and risk factors. This condition can affect different parts of the urinary tract and is influenced by factors like dietary deficiencies, urinary solute imbalances, and inadequate drainage. Various type
1 views • 58 slides
Understanding Osmosis and Cell Membrane Transport
Osmosis is the process of water molecules diffusing across a semi-permeable membrane, crucial for maintaining cell function. It involves the movement of water from areas of high concentration to low concentration until equilibrium is reached. Special proteins called aquaporins facilitate water trans
1 views • 24 slides
Preparation of Solution from Solid: Practical General Chemistry Techniques
Solutions are homogeneous mixtures where a solute is dissolved in a solvent. Known concentration solutions can be prepared using solid substances through careful techniques. This process involves weighing the solid, dissolving it in a beaker, transferring to a volumetric flask, and finalizing the so
0 views • 12 slides
Understanding Colligative Properties in Solutions
Colligative properties in solutions depend on the total concentration of solute particles present, impacting properties such as boiling point elevation, freezing point depression, vapor pressure lowering, and osmotic pressure. Boiling point elevation is directly proportional to the number of solute
1 views • 19 slides
Understanding Concentration of Solutions in Physiology
Concentration of solutions is crucial in understanding the properties of substances in Physiology. This involves concepts like percentage solutions and molar solutions, where the amount of solute is measured in grams or moles relative to the volume of the solution. Percentage solutions are commonly
0 views • 8 slides
Understanding Membrane Transport Processes in Plant Cells
Plant cells are separated from the environment by plasma membranes, which facilitate the movement of molecules through passive and active transport mechanisms. Transport proteins play a crucial role in allowing the movement of ions and polar molecules, with channels, carriers, and pumps enhancing so
0 views • 20 slides
Understanding Water Potential in Plant Physiology
Water potential is a crucial concept in plant physiology, driving the movement of water within plants. It is a measure of potential energy in water and influences processes like photosynthesis. This potential is influenced by factors such as solute concentration, pressure, gravity, and matrix effect
0 views • 25 slides
Understanding Osmosis in Cell Membranes
This quiz explores the concept of osmosis in cell membranes, focusing on the movement of water into outermost cells of roots by osmosis and its implications on cell sap. It covers topics such as active transport, diffusion, pressure in cell contents, and the role of water movement in maintaining the
0 views • 50 slides
Understanding Solution Concentration in Chemistry
Solutions in chemistry are homogeneous mixtures of two or more substances where each maintains its own chemical identity. They consist of a solute, the substance being dissolved, and a solvent, the substance used to dissolve the solute. Solution concentration can be described qualitatively as concen
0 views • 17 slides
Understanding Mixtures: Solutions, Colloidal Dispersions, and Suspensions
Food mixtures can be classified based on particle size distribution, with the dispersed phase scattered throughout a continuous medium. Solutions, a type of mixture, consist of a solute (dispersed phase) and a solvent (continuous phase), forming a homogenous blend. Factors like temperature, particle
0 views • 80 slides
Understanding Gravitational Potential Energy and Negative Potential Energy
Exploring the concept of gravitational potential energy beyond the surface of Earth, understanding how potential energy varies with distance from the Earth's center, and delving into the significance of negative potential energy at certain points. Learn about the calculations involved, including the
0 views • 17 slides
Understanding Freezing Point Depression and Molality for Solutions
Introduction to molality and freezing point depression in solutions. Molality is a way to measure solution concentration, calculated using moles of solute and kilograms of solvent. By calculating the moles of NaCl in a salt solution and the mass of the solvent (ice/water), the molality can be determ
0 views • 9 slides
Role of Solvent in Spectral Properties and Solvatochromism
Solvent plays a crucial role in physical and chemical processes, affecting kinetics, equilibria, and spectral properties such as UV-vis, IR, and NMR. Solvathochromism describes the change in spectral bands caused by solvent interactions. Factors like solvent polarity and hydrogen bonding influence d
0 views • 19 slides
Enhancing Food Preservation with Edible Coatings
Edible coatings provide a thin layer of material that acts as a barrier to oxygen, microbes, moisture, and solute movement for fruits and vegetables. They improve the appearance, shelf life, and nutritional composition of produce without compromising quality. Methods of applying edible coatings incl
0 views • 4 slides
Understanding Cell Environment Dynamics through Visual Charts
The provided content delves into the dynamics of cell environments, focusing on concepts like osmosis and solute concentration. Visual aids such as charts and images help illustrate how cells interact with their surroundings, highlighting processes like water movement, solute concentration changes,
0 views • 8 slides
Understanding Light Scattering Part 2: Solute Characteristics and Applications
Light scattering can provide valuable insights into solute characteristics such as molecular weight, radius of gyration, and second virial coefficient. By analyzing scattering data, information about individual particles and their arrangement in solution can be obtained. Techniques like Zimm or Guin
0 views • 20 slides
Understanding Osmotic Systems in Plant Cells
Plant cells function as osmotic systems where factors like solvent purity, solute concentration, and cell turgidity affect water movement. Osmotic pressure varies with factors like cell age, position, and plant habitat. Permeability of membranes, passage of substances, and laws of diffusion are cruc
0 views • 25 slides
Gas Chromatography: Behavior of Solute, Advantages, Disadvantages, and Applications
Gas chromatography involves the chromatographic behavior of solutes in various ways such as retention volume, retention time, and partition coefficient. It offers advantages like good separation, short analysis time, and universal detectors for organic compounds. However, there are disadvantages suc
0 views • 15 slides
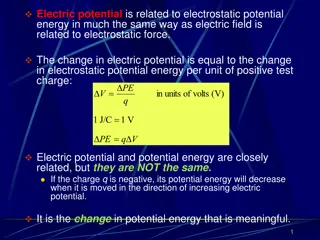
Understanding Electric Potential and Its Relationship to Electrostatic Energy
Electric potential is intricately linked to electrostatic potential energy, much like how electric field correlates to electrostatic force. The change in electric potential equals the change in electrostatic potential energy per unit positive test charge, expressed in volts. By exploring scenarios s
0 views • 24 slides
Understanding Adsorption Process in Physical Pharmacy Lab
Adsorption is a significant process in physical pharmacy involving the attachment of solutes or gaseous molecules onto the surface of a solid. This process can be strong or weak depending on the forces between the solid surface and the gas/solute. Types include physical (reversible) and chemical (ir
0 views • 16 slides
Pediatric CRRT Prescription: Rates, Dose, Fluids Overview
This informative content by Dr. Michael Zappitelli from Montreal Children's Hospital discusses the prescription rates, doses, and fluids involved in Pediatric CRRT. It covers insights on blood flow rates, diverse solutions used, and ideal compositions for Peritoneal dialysis fluid. The article empha
0 views • 20 slides
Understanding Molarity and Molality in Solutions
Solutions are combinations of solvent and solute, with varying concentrations affecting their properties. Molarity and molality are key measures of concentration, and saturation levels impact solubility. Explore examples to calculate molarity for solutions in different scenarios.
0 views • 12 slides
Understanding Colligative Properties in Chemistry
Colligative properties in chemistry depend on the amount and type of solute particles added to a sample, as well as the intermolecular forces at play. They include vapor pressure, boiling point elevation, and freezing point depression. Vapor pressure is the pressure exerted by a vapor on its surroun
0 views • 7 slides
Understanding Endothermic and Exothermic Reactions Through Experiment
Explore the concepts of endothermic and exothermic reactions with a detailed experiment to determine which solute dissolves most endothermically and exothermically in water. Discover the roles of heat transfer in chemical reactions using potassium chloride, calcium chloride, sodium carbonate, and so
0 views • 11 slides
Understanding Properties of Solutions: A Comprehensive Overview
Solutions are homogeneous mixtures of pure substances where the solute is uniformly dispersed in the solvent. This chapter delves into the formation of solutions, types of solutions (saturated, unsaturated, supersaturated), Henry's Law, the influence of temperature on solubility, and ways of express
0 views • 25 slides
Understanding Concentration and Dilution of Solutions by Dr. Laila Al-Harbi
Dr. Laila Al-Harbi explains the concept of concentration of solutions, focusing on molarity and the calculation involved. The molar concentration of solutions is determined by the amount of solute in a given volume of solvent, expressed as moles of solute per liter of solution. Additionally, the pro
0 views • 15 slides
Understanding Colligative Properties and Vapor Pressure in Solutions
Colligative properties are solution properties dependent only on solute concentration, not solute identity. The Van't Hoff factor helps in such equations, especially for ionic compounds. Vapor pressure lowering is a key colligative property where a solute decreases vapor pressure. Raoult's Law expla
0 views • 23 slides
Two-Dimensional Mathematical Model of Flows in Thin Film Composite Membranes
This study presents a mathematical model for flows in thin film composite membranes, focusing on the permeation of solvent flux and solute rejection. Assumptions include incompressible fluid, constant diffusion of chemical species, and isothermal conditions. Equations describe water flux, solute flu
0 views • 19 slides