Understanding Water Potential and Osmosis in Plants
This content delves into the concept of water potential, osmosis, and pressure potential in plant biology. It explains how water moves within plant cells, the unit of measurement for water potential, the role of osmotic potential in water movement, and the effects of solute concentration on water potential. Additionally, it discusses key terms like turgor pressure, plasmolysis, and flaccid state in plant cells.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
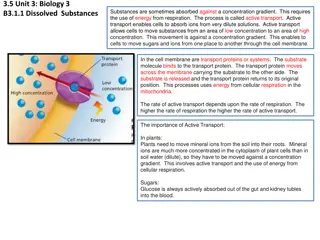
Water potential Osmotic potential Pressure potential Matric potential Absorption and translocation of water Stomatal regulation.
Difference b/w free energy of water in that system atmospheric temperature. Water potential is the potential energy of water per unit volume relative to pure water in reference conditions. Water potential quantifies the tendency of water to move from one area to another due to osmosis, matrix effects such as capillary action and free pressure energy of and pure water defined at a gravity, mechanical pressure and
Unit of measurement: Energy units, Joules per m3, Pascals Pure water =0 Adding solute lowers potential Less free water molecules Water moves from a higher water potential to a lower water potential Less concentrated (hypotonic) to a more concentrated (hypertonic)
Osmosis Movement water from a higher potential region water through a semi- permiable membrane. Osmosis Diffusion Net movement from one point to another because of random kinetic activities of molecules from a region of their own concentration region of their lesser concentration. It process. Diffusion of molecules region of water to of potential or ions higher to a lower a is spontaneous
Water potential = Solute potential + Pressure potential = s + p Osmotic potential Solute potential Pressure that a solution have to build to increase its chemical potential to that of pure water. Solute potential is always negative. Osmotic potential Pressure potential Equivalent to pumpimg water from one place to another. If pressure greater than atmospheric pressure is applied to pure water or a solution, its water potential increases. Pressure potential Water potential Tendency of water to leave the system Higher water potential, greater tendency to leave Water passes from one system to the other, through membrane by osmosis Water potential
Flaccid: Turgid: Plasmolysis: Plant cell shrinks from cell wall Lost water Deplasmolysis: Plant cell resumes turgidity Gained water Limp-lost water Firm-gained water
Environment: 0.01 M sucrose 0.01 M glucose 0.01 M fructose Cell 0.03 M sucrose 0.02 M glucose
(a) 0.1 M solution Pure water H2O P= 0 S= 0 = 0 MPa P= 0 S = 0.23 = 0.23 MPa
(b) Positive pressure H2O P= 0.23 S = 0.23 = 0 MPa P= 0 S= 0 = 0 MPa
(c) Increased positive pressure H2O P = S = 0.23 = 0.07 MPa P= 0 S= 0 = 0 MPa 0.30
Initial flaccid cell: P= 0 S = 0.7 = 0.7 MPa 0.4 M sucrose solution: P= 0 S = 0.9 = 0.9 MPa Plasmolyzed cell P= 0 S = 0.9 = 0.9 MPa (a) Initial conditions: cellular > environmental
Initial flaccid cell: P = 0 S = 0.7 = 0.7 MPa Pure water: P = 0 S = 0 = 0 MPa Turgid cell P = 0.7 S = 0.7 = 0 MPa (b) Initial conditions: cellular < environmental
s = -iCRT i = ionization constant Sucrose=1.0 (sucrose does not ionize water) C = Molar concentration (from experiment) R = Pressure constant (R=0.0831liter bars/mole K) T = temperature in K (273 + C)