Understanding Osmosis and Cell Membrane Transport
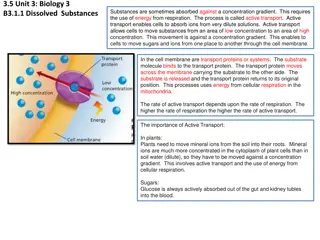
Osmosis is the process of water molecules diffusing across a semi-permeable membrane, crucial for maintaining cell function. It involves the movement of water from areas of high concentration to low concentration until equilibrium is reached. Special proteins called aquaporins facilitate water transport across cell membranes. By assessing solute and solvent concentrations, we can predict osmosis direction and its effects on cells based on tonicity – hypotonic, hypertonic, or isotonic environments.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Osmosis is the diffusion of water molecules happens across a semi-permeable membrane
ONLY WATER Water is a small but extremely important molecule that makes up most of the liquid part of the cytoplasm in living things. Deals ONLY with the diffusion of WATER The molecules (in this case, water - not solute molecules) will tend to move from an area of high (water) concentration to an area of low(water)concentration until equilibrium is reached.
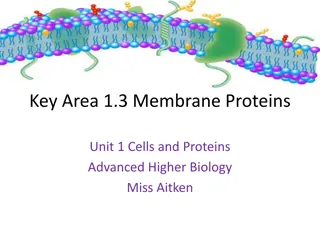
OSMOSIS: FACILITATED DIFFUSION OF WATER ACROSS A CELL MEMBRANE Why would water molecules normally have a hard time getting across the cell membrane? The inside of a cell s lipid bilayer is hydrophobic (water hating) Click me! Click me!
Aquaporins Most cells have special water channel proteins Known as Aquaporins Allow H2O to pass right through them by facilitated diffusion. This EXTREMELY important process is = OSMOSIS
By knowing the concentrations of solute and solvent on the inside and outside of a cell, we can predict the direction of osmosis and the result on the cell. Solutions on the outside of a cell can be described based on how they affect the cell hypERtonic hypOtonic isotonic
Solutions on the outside of a cell (in its environment) can be described based on how they affect the cell: Hypotonic Hypertonic Isotonic Above Strength Cell Shrinks The solution outside the cell has a [higher] of solutes than inside Water moves OUT of cell Below Strength Cell Swells The solution outside the cell has a [lower] of solutes than inside Water moves INTO the cell Same Strength Cell stays the same the solution outside the cell has the [SAME] of solutes than inside Water moves EQUALLY in/out of the cell NOTE: (*tonic = solute. [High] solute means [low] water) "HYPER" = HIGH; "HYPO" = LOW; "ISO" = equal or same.
VISUALIZE HYPERTONIC What will happen? a) net movement of water _______of cell Cell with 2% solute, 98% solvent Beaker with 3% solute, 97% solvent b) cell will ________ H2O c) solution is hypertonic to the cell H2O H2O AFTER BEFORE
VISUALIZE HYPOTONIC What will happen? a) net movement of water _______of cell Cell with 2% solute, 98% solvent Beaker with 1% solute, 988% solvent b) cell will ________ H2O c) solution is hypotonic to the cell H2O H2O AFTER BEFORE
VISUALIZE ISOTONIC Cell with 2% solute concentration, 98% solvent Beaker = 2% solute, 98% solvent What will happen a) no net movement b) cell won t change in size c) solution = isotonic to the cell
Common mistakes when discussing hyper-, hypo-, and isotonic solutions The solutions are named for the concentrations of the SOLUTES The substance that moves to balance the solute concentration is the WATER The solutes to not pull or suck the water across the membrane the water simply diffuses from where it is in high concentration to low concentration
Solute and solvent concentrations can be expressed as percentages of the entire solution. When added together, the solute and solvent concentrations must equal 100%. A solution with a 10 % solute concentration has a 90% solvent concentration.
Lets do some math! What is the solvent concentration of a solution with a 3% concentration of solute? What is the solute concentration of a solution with 98% solvent? What is the solute concentration of a solution with 75% water? What is the solvent concentration of a solution with a 15% concentration of glucose?
OSMOTIC PRESSURE Driven by differences in solute concentration, the net movement of water into or out of a cell produces a force known as osmotic pressure
Almost always hypertonic Because cells contain a variety of solutes such as: sugars, proteins, salts, etc. they are almost always hypertonic (*the environment = HYPOtonic!) to fresh water; as a result, a typical cell exposed to fresh water will tend to swell up quickly from the entering water. This may in fact cause an animal cell to swell like an overinflated balloon.
Plant cells contain a central vacuole which stores excess water - shrinking and swelling as water enters or exits the cell. Plant cells wouldn't generally burst thanks to their protective cell walls. In fact, most cells in large organisms are not in contact with fresh water on a regular basis - rather, they tend to be bathed in blood or other isotonic fluids which have solute concentrations approximately equal to themselves. Cells which are plump and rigid in hypotonic environments are called turgid; when a cell shrinks in a hypertonic environment this is called plasmolysis
Fill in Conditions Water will Environment is... Move in and out in equal amounts No net movement Isotonic to the cell Cell is isotonic to its environment Solute concentration in the environment is equal to that in the cell Move OUT of the cell The Cell Shrinks Hypertonic to the cell Cell is hypotonic to its environment Solute concentration in the environment is greater than the cell Solute concentration in the environment is less than the cell Move INTO the cell The Cell Swells Hypotonic to the cell Cell is hypertonic to its environment
Show what happens to plant and animal cells subjected to isotonic, hypertonic, and hypotonic solutions in the environment: Conditions Environment Plant Cell (leaf cell) Before Animal cell (blood cell) Before After After Solute concentration in the environment is equal to that in the cell: Isotonic solution Solute concentration in the environment is greater than the cell: Hypertonic Solution Solute concentration in the environment is less than the cell: Hypotonic Solution
APPLY what you have learned about osmosis Why do doctor s use a saline solution in an IV drip?
APPLY what you have learned about osmosis Why would salt kill plants?
APPLY what you have learned about osmosis Why do restaurants put out free salty snacks such as peanuts, pretzels or chips?