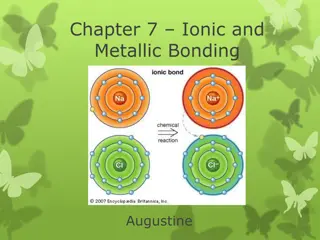
Understanding Ionic and Metallic Bonding: Valence Electrons, Octet Rule, and Ion Formation
Explore the essential concepts of ionic and metallic bonding, focusing on valence electrons, electron dot structures, the octet rule, cations, anions, and ion formation. Discover how atoms achieve stability through electron transfer, and learn to write electron configurations for various ions.
9 views • 52 slides
Understanding Ionic and Metallic Bonding in Chemistry
Explore the concepts of ionic and metallic bonding in chemistry through discussions on valence electrons, electron dot structures, the octet rule, cations, anions, and more. Dive into the world of ions and electron configurations to understand how atoms achieve stability through the gain or loss of
3 views • 62 slides
Understanding Ionic and Covalent Bonding in Chemistry
Ionic bonding involves the transfer of electrons between a metal and a non-metal to form a giant lattice structure, like in sodium chloride and lithium oxide. Covalent bonding, on the other hand, occurs between non-metals, resulting in giant covalent structures or simple molecules. Examples such as
4 views • 79 slides
Philosophy of Thales: The Ancient Wisdom of the Ionic School
Thales of Miletus, a key figure in the Ionic School of philosophy, is considered the father of philosophy. He believed that water was the principle of all things and made significant contributions to mathematics and astronomy. Thales's wisdom and engineering feats, such as diverting the river Halys,
0 views • 18 slides
Understanding Chemical Bonds and Ionic Compounds
Ionic bonds are formed when atoms transfer electrons to achieve stable electron configurations, resulting in the creation of ions with positive or negative charges. Metals are good conductors due to their ability to easily lose electrons. The charges of ions depend on the number of valence electrons
0 views • 49 slides
Understanding Ionic and Metallic Bonding in Chemistry
Explore the concepts of ions, electron dot structures, the octet rule, cations, and anions in Chapter 7. Learn how elements achieve stability through electron configurations, and practice writing electron dot structures and naming ions. Understand the differences between cations and anions and how t
1 views • 52 slides
Understanding and Utilizing the Bursting Strength Tester: A Comprehensive Guide
In the world of material testing, ensuring the strength and durability of products is crucial. One essential tool that plays a pivotal role in this process is the bursting strength tester. This device helps manufacturers and quality control professionals measure the bursting strength of various mate
6 views • 5 slides
Testing Methods for Textile Fabrics Breaking Strength and Elongation
Standard testing methods, ASTM D5034 and D5035, are used to determine the breaking strength and elongation of textile fabrics through grab and strip tests. The grab test measures the effective strength of fabric using adjacent yarns, while the strip test compares the strength of unwoven yarns. Appar
0 views • 29 slides
Understanding Strength-Duration (SD) Curve: A Neuromuscular Diagnostic Test
Strength-Duration (SD) curve is a diagnostic test assessing neuromuscular integrity by measuring the relationship between stimulus strength and duration. It helps in electro-diagnosis of peripheral nervous system disorders and evaluates nerve degeneration and regeneration. The curve is obtained by p
2 views • 17 slides
Chemistry Concepts: Valence Electrons, Ion Charges, and Ionic Compounds
Explore various key concepts in chemistry such as valence electrons in magnesium, Lewis Dot structure for silicon, charges on ions like strontium, formation of ions to achieve noble-gas electron configuration, elements forming ions with specific charges, and the octet rule. Learn about the character
1 views • 48 slides
Understanding Ionic Bonding and Lattice Energy
Explore the world of ionic bonding through images and explanations. Learn how electrons are transferred to form ions, the arrangement of ions in a crystal lattice, and the concept of lattice energy in ionic compounds. Discover the formation of formula units, examples of bond pairs, and the significa
1 views • 18 slides
ASTM D3786/D3786M-18 Standard Test Method for Bursting Strength of Textile Fabrics
This standard test method, ASTM D3786/D3786M-18, specifies the procedure for determining the bursting strength of textile fabrics using a diaphragm bursting strength tester. The test involves clamping the specimen over an expandable diaphragm and applying pressure until the fabric ruptures. The burs
0 views • 16 slides
Rejuvenate with Ionic Foot Detox in New Jersey
Experience the benefits of ionic foot detox at Zen Health & Wellness Center in New Jersey. Detoxify, relax, and rejuvenate for improved overall well-being. Book your session today.\nFor more information:\nCall us: 8564620579\nMail us: ZENHEALTHANDWEL
4 views • 8 slides
Understanding Pharmaceutical Product Strength Alteration
Exploring methods to adjust the strength of pharmaceutical preparations by manipulating the proportion of active ingredients, dilution, concentration, and the relationship between strength and total quantity. Learn about solving problems related to altering product strength using inverse proportion,
0 views • 17 slides
Understanding Ion-Pair Chromatography (IPC): Theory and Applications
Ion-Pair Chromatography (IPC) involves adding ionic surfactants to a reversed-phase Chromatography system to affect retention and selectivity of ionic compounds. Developed by Dr. Gordon Schill, IPC is crucial for resolving hydrophilic samples and controlling selectivity in separations. The theory in
7 views • 18 slides
Understanding Pharmaceutical Preparations: Strength and Concentration
Pharmaceutical preparations can have their strength and concentration adjusted by changing the proportion of active ingredients. This process involves increasing or decreasing the active ingredient, adding diluents, admixing with other preparations, or evaporating the vehicle. Dilution of liquid, so
0 views • 34 slides
Chemical Bonding and Compound Formulas: Understanding Ionic vs. Covalent Bonds
Explore the differences between ionic and covalent bonds, learn about ionic compounds held by electromagnetic attractions, understand molecular compounds with shared electrons, and grasp the naming conventions for ions. Discover how molecular formulas and formula units represent atoms in compounds.
0 views • 56 slides
Understanding Protein Solubility: Effects of pH, Ionic Strength, and Salting Out
Exploring the factors influencing the solubility of proteins, including pH levels, ionic strength, and salting out techniques. This study delves into the isoelectric point of proteins and the impact of salt addition on solubility, aiming to enhance comprehension in the field of protein biochemistry
0 views • 14 slides
Chemistry: Naming Compounds and Writing Formulas
Understand compounds, chemical formulas, and how to write ionic formulas using the Swap 'n Drop Method. Learn about types of compounds - ionic, covalent, and acids, and practice writing formulas for various elements. Follow rules, naming flow charts, and partner activities to enhance your understand
0 views • 13 slides
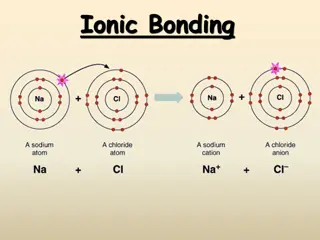
Understanding Ions, Ionic Bonds, and Ionic Compounds
Ions are charged particles formed by gaining or losing electrons, leading to the formation of ionic bonds between positively charged cations and negatively charged anions. The octet rule guides electron configurations, and the periodic table helps predict ion formation. Ionic bonding involves electr
0 views • 13 slides
Understanding Cardiac Action Potential: Ionic Basis and Excitability in Cells
Explore the complex processes underlying cardiac action potential, from ionic equilibrium to resting membrane potential and excitability in cardiac cells. Learn about the critical thresholds, equilibrium potentials, and gradients that regulate the electrical activity of the heart. Discover the intri
0 views • 68 slides
Understanding Ionic Compound Formulation and Nomenclature
Ionic compounds consist of cations and anions combined in simple ratios to balance charges. The cross technique helps determine the correct ratio of ions. Reduction of ratios may be necessary in certain cases. Formulation and naming examples are provided to illustrate these concepts.
1 views • 12 slides
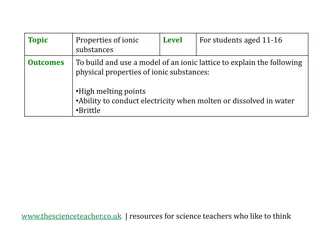
Exploring Physical Properties of Ionic Substances through Model Building
Engage students aged 11-16 in an interactive activity to understand the physical properties of ionic substances such as high melting points, ability to conduct electricity, and brittleness. By building a model of an ionic lattice for sodium chloride and explaining how the structure relates to these
0 views • 5 slides
Understanding Chemical Bonding and Atomic Properties
Explore the formation of ionic and covalent bonds, electron configurations of ions, and molecular geometry. Learn about ionic compound formation, atomic properties like effective nuclear charge, atomic size, ionization energy, and electron affinity. Discover the essential concepts of cations formati
1 views • 73 slides
Total Body Push Pull Rotate Training for Strength and Endurance
Total Body Push Pull Rotate Training helps improve muscular endurance and strength by alternating exercises from different strength training categories. The workout consists of choosing exercises from push/pull categories and incorporating rotational movements for a comprehensive full-body workout.
0 views • 5 slides
Understanding Electrolytes and Ionic Equilibrium in Chemistry
Electrolytes play a crucial role in conducting electricity through ionization in aqueous solutions, with examples of strong and weak electrolytes explained. The concept of ionic equilibrium, degree of dissociation, and distinction between non-electrolytes are also covered in this comprehensive overv
0 views • 21 slides
When I Am Weak, Then I Am Strong: Understanding Strength in Weakness
In 2 Corinthians 12:6-10, the apostle Paul reflects on how a thorn in the flesh humbled him, leading to a greater understanding of God's grace and strength in weakness. This passage emphasizes that true strength is found in acknowledging our dependence on Christ, allowing His power to be perfected i
0 views • 7 slides
Understanding Ceramic Properties: Strength, Brittle Behavior, and More
Ceramics exhibit specific properties such as high compressive strength, brittleness due to mixed ionic-covalent bonding, low fracture toughness, poor electrical and thermal conduction (except for some types), and chemical insensitivity. While strong in compression, ceramics are brittle and lack duct
0 views • 13 slides
Understanding Ionic and Molecular Compounds in Chemistry
Discover the fundamental concepts of ionic and molecular compounds in chemistry with insights into the nature of elements, formation of compounds, and properties of ions. Explore the differences between ionic and covalent bonds, positive and negative ions, as well as examples of common everyday comp
0 views • 54 slides
Understanding Chemical Bonds: Covalent, Ionic, and Metallic
Explore the fascinating world of chemical bonds, including covalent bonds where atoms share electron pairs (e.g., water), ionic bonds where oppositely charged ions attract (e.g., sodium chloride), and metallic bonds formed between positively charged atoms sharing free electrons (e.g., copper wire).
0 views • 6 slides
Understanding Plain & Reinforced Concrete Structures in Design Engineering
In the design of Plain & Reinforced Concrete structures, various strength design methods such as Ultimate Strength Design (USD) and Allowable Strength Design (ASD) are utilized. These methods involve factors of safety, material strength, load factors, and analysis in the elastic range. Additionally,
0 views • 11 slides
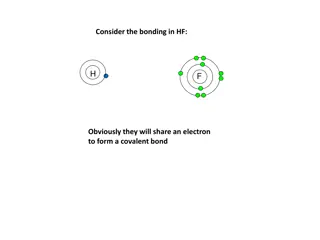
Understanding Bonding in HF Molecule
In HF bonding, hydrogen and fluorine share an electron to form a covalent bond. Fluorine, being more electronegative, attracts the bonding electrons more, resulting in a polar covalent bond. If hydrogen was less electronegative, the bonding electrons would shift further towards fluorine until an ion
0 views • 11 slides
Understanding Naming Ionic Compounds and Formulas
Ions and salts play a crucial role in forming solid compounds known as salts. By following specific rules, you can name ionic compounds based on the cation and anion present. Determining formulas involves identifying charges and balancing them. Explore the process through examples like KCl, MgO, AlC
0 views • 11 slides
Understanding Ionic Bonding and Octet Rule in Chemistry
Understanding the concept of ionic bonding and octet rule in chemistry is essential for grasping how atoms combine to form molecules through sharing or exchanging electrons. This process involves the formation of positive and negative ions held together by electrostatic attraction, leading to the cr
0 views • 12 slides
Understanding Ionic Bonding and Lattice Energy in Chemistry
Chemical bonds play a crucial role in holding atoms together in molecules. This course explores the concept of chemical bonding, focusing on ionic bonds and lattice energy. Topics covered include the different types of chemical bonds, such as electrovalent and coordinate bonds, as well as the models
0 views • 22 slides
Understanding Solid State Chemistry: Principles and Classification of Solids
Solid State Chemistry (CHEM 422) explores the principles and concepts governing the synthesis, structure, bonding, reactivity, and properties of solid state materials. The course delves into crystalline vs. amorphous solids, highlighting categories like ionic, molecular, metallic, and covalent solid
0 views • 24 slides
Understanding Ionic Bonding and Naming in Chemistry
Exploring the fundamentals of ionic bonding, naming conventions, and the Octet Rule in chemistry. Learn about Lewis structures, formation of ionic compounds, and the role of valence electrons in determining chemical properties. Discover how elements gain or lose electrons to achieve a full outer ene
0 views • 24 slides
Understanding Bonding in Ionic, Covalent, and Metallic Compounds
Explore the concepts of ionic, covalent, and metallic bonding through an investigation conducted by Vanderbilt Student Volunteers for Science. Learn about the different types of bonding, properties of ionic and molecular compounds, and the conductivity of metals. Discover the importance of determini
0 views • 15 slides
Understanding Concrete Strength in Structural Design
Concrete strength, including compressive, tensile, and flexural strengths, plays a crucial role in structural design. Compressive strength is the main quality criterion, while tensile strength is lower due to concrete's brittleness. Flexural strength also impacts the behavior of structural members.
0 views • 17 slides
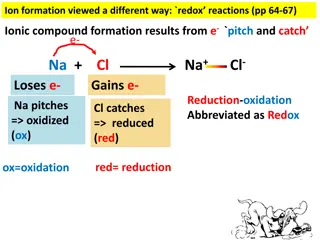
Understanding Redox Reactions and Ionic Compound Formation
Explore the concept of redox reactions through the process of ion formation, where elements lose or gain electrons to create ionic compounds. Learn about oxidation (ox) and reduction (red) in chemical reactions, and how to identify which elements lose or gain electrons based on charge changes. Disco
0 views • 14 slides