Course Registration for SPRING 2023
Attention AUCA freshmen! Online course registration for Spring 2023 is from November 21 to December 2. Make sure to complete the Anti-Harassment Training to be eligible for registration. Watch the registration tutorial and follow your department's checklist for a smooth registration process. Underst
2 views • 37 slides
REGISTRATION OF SHIPS UNDER MALTESE LAW
Explore the comprehensive process and legal framework for the registration of ships under Maltese law explained by Christian Farrugia, LL.M, LL.D. Learn about the registration of assets, ship nationality, ownership requirements, eligible vessels, and the registration process. Understand the signific
2 views • 21 slides
Introduction to Flood Risk Assessment with HEC-FDA Overview
This presentation delves into flood risk assessment using HEC-FDA software, covering topics such as defining flood risk, components of uncertainty, consequences of flood risk, and methods to assess flood risk including hydrology, hydraulics, geotechnical, and economics. It explores the intersection
6 views • 39 slides
PM Vishwakarma Registration Process Flow
The process flow for registering applicants for PM Vishwakarma involves gathering necessary information, accessing the PM Vishwakarma portal, and completing registration via the CSC/VLEs. Applicants need to provide Aadhaar details, ration details, savings account details, and other key information t
0 views • 13 slides
SACE Presentation on Registration Requirements
The presentation by Ms. Yvonne Lechaba, Head of Registration & Teacher Professionalisation at SACE, focuses on the registration requirements for educators in South Africa according to the SACE Act. It outlines the vision, mission, and values of SACE, as well as the professional registration value ch
5 views • 13 slides
Voter Registration Process in New York State: A Comprehensive Guide
Learn how to run a successful voter registration drive in New York State with this detailed guide. Explore who qualifies to register, how New Yorkers can register online, in-person, or by mail, what is needed for registration, and the mandatory questions on the registration form. Get insights into o
1 views • 19 slides
Understanding GST Registration Essentials
Dive into the intricacies of GST registration with a focus on different types of registration, eligibility criteria, exemptions, and aggregate turnover calculations. Gain insights from expert CA Indranil Das on key topics and practical issues related to GST registration. Unravel examples, explore pe
4 views • 42 slides
Voter Registration Process in Colorado
Learn about voter registration in Colorado, including eligibility requirements, automatic voter registration process, new registration statistics, and online voter registration. Discover who is eligible to register, how automatic registration works, and the options available to voters in the state.
2 views • 35 slides
Tatmeen Users Training - User Registration Process Overview
This document provides an overview of the user registration process for Tatmeen users, focusing on the registration of Single Point of Contact (SPOC) and non-SPOC users. It includes details on how users receive registration invitations, the different user types, steps for SPOC registration, data flo
2 views • 20 slides
Strategies for Accelerated Death Registration in Kenya: Insights from Janet Mucheru
The Civil Registration Services in Kenya faces challenges in death registration, but through lessons learned, best practices are recommended for accelerated progress. The department's core functions, purpose of civil registration, and death registration processes in Kenya are outlined, shedding ligh
3 views • 12 slides
Understanding the FDA Approval Process for Medical Devices
The FDA approval process for new medical devices involves rigorous evaluations to ensure safety and effectiveness. Conflicting criticisms of the FDA focus on the balance between tightening or loosening regulations without compromising public health. The agency's mission emphasizes protection and adv
0 views • 19 slides
fda registration
IAS helps organizations to register their products in US FDA. It is always a tedious process to collect and file the application as the US-FDA has got stringent rules. We are having experience in US-FDA registration thus we put forward our Services t
3 views • 2 slides
Student Planning Self-Registration Guide for Term 2: Important Dates and Steps
Understand the process of self-registration using Student Planning for Term 2, access registration dates, find where to locate Student Planning in HUB, and follow essential instructions provided via email for successful registration. Learn about planning courses, schedules, electives, and registrati
0 views • 15 slides
CSM.ASLP Registration Guidance for Implementing Users
This guidance provides detailed instructions on the registration process for users implementing the CSM.ASLP requirements. It covers the importance of registration, preparing for quality registration, entity contacts, filling out registration templates, and contacting ERA. Key points include the pur
0 views • 16 slides
Voter Registration Responsibilities and Procedures at MVD Offices
Presented by Raul Alvarez at the 2010 Managers Conference, this information outlines the key responsibilities for Voter Registration Contacts at MVD offices. It includes details on the importance of having designated contacts, handling voter registration forms, and adhering to procedures to offer vo
0 views • 21 slides
Vehicle Inspection and Registration System Overview
This information highlights the changes in vehicle inspection and registration processes, particularly focusing on the transition to a registration-based enforcement system starting March 1, 2015. It explains the importance of the Vehicle Inspection Report (VIR) and how the new system affects regist
0 views • 21 slides
PhytClean.V2 Citrus 2022/2023 Special Market Registration Update
This update covers the changes in the Citrus 2022/2023 Special Market Registration process using PhytClean.V2. It includes a walkthrough of the registration system, information on registration periods, costs, available markets, dashboard features, and document requirements. Seasonal registration rem
0 views • 9 slides
Step-by-Step Guide for Connect & Canvas Student Registration
Step 1: Enter your Canvas username and password, then click Login to start the registration process. Step 2: Navigate to Courses and select a course name. Step 3: Access Assignments from the course home page. Step 4: Click on the Connect assignment. Step 5: Begin the assignment. Step 6: Register as
1 views • 13 slides
Juvenile Sex Offender Registration and Mandatory Offenses
The content details the requirements and processes related to juvenile sex offender registration, including discretionary removal from the registry, comprehensive evaluations, and mandatory registration for specific offenses. It also lists the mandatory offenses that require registration for juvenil
1 views • 21 slides
Understanding Voter Registration and Updates
Discover how voter registration works, eligibility criteria, methods of registration, deadlines, and the role of election officials. Learn about the National Voter Registration Act and the importance of maintaining accurate voter lists for safeguarding voters. Get insights into the processes involve
0 views • 16 slides
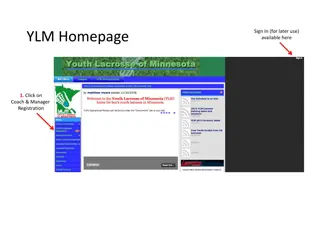
Step-by-Step Guide for Coach and Manager Registration
This detailed guide provides step-by-step instructions for Coach, Assistant Coach, and Manager Registration on the YLM homepage. Follow the outlined process to sign in, create an account, choose your team, and complete the registration. Coaches and managers are required to have specific information
0 views • 5 slides
Civil Registration System in Uganda: Legal Framework and Responsibilities
The civil registration system in Uganda is governed by a legal framework including the Constitution, Registration Services Bureau Act, Children Act, and other laws. The Uganda Registration Services Bureau oversees the registration of vital events such as births, deaths, marriages, and adoptions. Reg
0 views • 23 slides
Examples of Real-World Evidence in Regulatory Decisions by FDA
FDA showcases illustrative examples of how Real-World Evidence (RWE) has been utilized in regulatory decisions, highlighting its value across different submission types, data sources, and purposes from FY12-FY19. The FDA report features cases like a modified hemodialysis catheter end cap, tumor prof
0 views • 14 slides
Civil Registration of Acts in Russia
Civil registration of acts in Russia is governed by key legislation including Federal Law No. 143-FZ and involves the state registration of various events such as birth, marriage, divorce, and death. Bodies responsible for registration are formed by public authorities of the Russian Federation, with
0 views • 24 slides
Unregulated Risks of E-Cigarettes: FDA and Safety Concerns
E-cigarettes, unregulated and not FDA-approved, pose health risks with potential carcinogens and toxic chemicals. Lack of oversight allows for manufacturers to sell products with varying safety standards. FDA testing has revealed concerning findings, yet regulations are limited in addressing key iss
0 views • 40 slides
Understanding Texas Boat Registration and Title Search Guidelines
Boaters in Texas need to be aware of registration requirements and title search guidelines. The Parks and Wildlife Department issues boat registration numbers starting with "TX" and both titles and registration stickers. Knowing about hull ID numbers, liens, and how to look up registration records i
0 views • 10 slides
Expert Tips for Successful FDA Inspections
Gain insights from Joyce Nancarrow Tull, an experienced professional, on navigating FDA inspections successfully. Learn about legal authority, inspection procedures, key strategies, and more. Be proactive, seek expert advice, and build knowledge to enhance your readiness for FDA audits.
0 views • 31 slides
Understanding the Structure and Functions of FDA in the USA and Canada
The Food and Drug Administration (FDA) in the USA is a critical agency within the Department of Health and Human Services responsible for regulating the safety of various products such as foods, drugs, medical devices, and cosmetics. The FDA has distinct organizational units like the Office of the C
0 views • 49 slides
Reform Efforts in Civil Registration and Vital Statistics System in Tanzania
The Registration Insolvency and Trusteeship Agency (RITA) in Tanzania is responsible for vital event registration, insolvency services, and trusteeship services. The history of birth registration in Tanzania dates back to the colonial era, with separate systems for Mainland Tanzania and Zanzibar. La
0 views • 9 slides
Streamlining Registration and Enrollment Process Overview
This detailed guide provides essential information on registration and enrollment procedures, emphasizing the importance of training multiple personnel at each school. Key topics covered include new student registration, returning student registration, Parent Portal access, system differences betwee
0 views • 21 slides
BRAVO! Birth Registration Impact Evaluation Study in Burkina Faso
BRAVO! (Birth Registration for All Versus Oblivion) is a program collaborating with governments in Burkina Faso, Malawi, and Mozambique to link civil registration to health systems. The impact evaluation study in Burkina Faso's Sangui province shows the positive effects of opening stable registratio
0 views • 10 slides
Strategies for Accelerated Progress in Birth Registration in Namibia
Namibia has made significant strides in improving birth registration processes over the past decade, focusing on comprehensive assessments, strategic planning, electronic systems, collaborations, and research. Key strategies include high-level commitment, electronic notification systems, hospital-ba
0 views • 7 slides
Understanding FDA Regulations in Clinical Research
FDA regulations in clinical research cover the protection of human subjects, IND/IDE requirements, risk determinations, application processes, and what falls under FDA regulation. It distinguishes between FDA-regulated activities and those not regulated, such as medical record reviews. The scope inc
0 views • 31 slides
Bioanalysis Validation Case with Michael Cohen-Wolkowiez at Duke University
Michael Cohen-Wolkowiez, MD PhD, a Pediatric Trials Network Professor at Duke University, conducted a bioanalysis validation case study involving Meropenem in infants funded by NIH. The study focused on PK and safety data for FDA registration consideration. High-level results showed significant diff
0 views • 6 slides
Off-Label Marketing and Free Speech in the Post Caronia and Amarin World
Background of off-label use and prohibition, First Amendment challenges to FDA authority, next steps for industry and FDA in responding to the new legal landscape. FD&C Act prohibits misleading labeling, FDA's regulatory authority on drug and device promotions, FDA's view on off-label promotion proh
0 views • 19 slides
Procedures and Management of Pesticide Registration in Nepal
This content provides detailed information on the registration procedures, management, and regulation of pesticides in Nepal. It covers the registration method, advantages of analogous registration, banned pesticides list, types of pesticides, and essential documentation requirements for pesticide r
0 views • 43 slides
Amendments and Changes in Lobbyist Registration Requirements
Amendments to the definition of legislative persons, inclusion of certain individuals and organizations in the definition, changes in registration fees, and shifts in the registration year timeline for lobbyists in Indiana starting from July 1, 2013. The process for filing annual registration statem
1 views • 24 slides
Records and Registration Training for Department Chairs
This training module for department chairs covers various topics such as reinstatement, academic activity tracking, course withdrawal limits, petitions and forms, academic misconduct, grading, suspension processing, FERPA, prerequisites, registration restrictions, registration overrides, and more. I
0 views • 40 slides
GCSG 2022 US Conference Vendor Pre-Registration Information
Detailed information about the GCSG 2022 US Conference Vendor Pre-Registration process, including important dates, registration requirements, sponsorship opportunities, and contact details for queries or concerns. The registration includes a 1-year membership to GCSG for access to conference informa
0 views • 33 slides
Voter Registration Guide for Registration Officers in Botswana
Dive into an informative presentation outlining the Electoral Act, qualifications, and importance of voter registration in Botswana. Get insights on duties, legal frameworks, and procedural aspects essential for Registration Officers (ROs) in managing voter registration effectively.
0 views • 25 slides