Understanding Alinity i STAT High Sensitivity Troponin-I for Cardiovascular Diagnosis
Alinity i STAT High Sensitivity Troponin-I is a proprietary assay used in diagnosing myocardial infarction (MI) by quantitatively measuring cardiac troponin I in human plasma. This chemiluminescent microparticle immunoassay (CMIA) aids in the detection of MI, a significant aspect in managing heart d
2 views • 30 slides
Aspirin Assay by Direct Acid-Base Titration Experiment Overview
Exploring the process of assessing aspirin purity through direct acid-base titration using sodium hydroxide as a standard solution. The experiment includes details on aspirin properties, dosage, acidity, decomposition, and metabolism. Key aspects covered include the aim of the experiment, the princi
5 views • 15 slides
Assay of Ascorbic Acid (Vitamin C) - Method and Procedure
Ascorbic acid, known as Vitamin C, is a crucial organic compound with antioxidant properties. This article discusses the assay of ascorbic acid using a redox titration method with potassium iodate and iodide. The principle, procedure, and calculations involved in determining the concentration of Vit
1 views • 8 slides
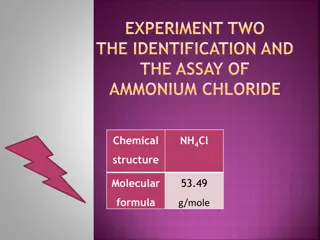
Identification and Assay of Ammonium Chloride: Experiment Insights
Explore the identification and assay process of Ammonium Chloride (NH4Cl), a weak inorganic acid commonly used in various applications. Discover its chemical properties, synthesis, reactivity, identification tests, and details of a titration process using NaOH and phenolphthalein as indicators.
0 views • 16 slides
Understanding Evaluation and Validation Methods in Machine Learning
Classification algorithms in machine learning require evaluation to assess their performance. Techniques such as cross-validation and re-sampling help measure classifier accuracy. Multiple validation sets are essential for comparing algorithms effectively. Statistical distribution of errors aids in
0 views • 95 slides
Revolutionary N-Assay: Transforming Pathogen Detection in Healthcare
Innovative N-Assay developed by Jonathan Faro, MD, PhD, offers a faster, more accurate, and cost-effective solution for detecting bacterial pathogens in healthcare settings. Unlike traditional assays, N-Assay differentiates between viable and non-viable pathogens, provides information on antibiotic
1 views • 15 slides
Approval and Validation Updates for NR MIMO OTA in 3GPP TSG-RAN.WG4 Meeting
The 3GPP TSG-RAN.WG4 Meeting discussed approval for the NR MIMO OTA workplan, addressed FR2 blocking issues, and emphasized the use of polarized antenna models. Updates were made to the power validation procedure and gNB beams usage criteria for FR1 MIMO OTA channel model validation. Collaboration w
1 views • 8 slides
Principles of Calibration, Validation, and Warehousing in Pharmaceutical Quality Assurance
Calibration and validation are critical processes in maintaining the quality of pharmaceutical products. Validation ensures that processes result in expected outcomes consistently, meeting quality standards. Qualification is an essential part of validation, ensuring that equipment and systems perfor
1 views • 37 slides
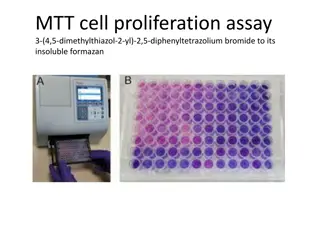
MTT Cell Proliferation Assay: Protocol and Applications
The MTT cell proliferation assay is a widely used technique to measure cell viability, proliferation, and cytotoxicity in research. This colorimetric assay involves the conversion of MTT reagent to formazan dye by live cells, resulting in a purple color change. This comprehensive guide provides step
0 views • 8 slides
Ortho Vision Validation and Operation in RCI
Role of RCI laboratory, analyser requirements, validation process, installation verification, and PQ testing for Ortho Vision system. The RCI laboratory plays a crucial role in various testing processes including blood grouping, antibody ID, and compatibility testing. Validation process includes URS
0 views • 17 slides
Understanding Clinical Validation and DRG Validation in Healthcare
Clinical validation ensures that diagnoses documented in a patient's record align with accepted clinical criteria, while DRG validation focuses on matching hospital-coded information with physician descriptions and patient records. Clinical validation involves a review by clinicians to confirm the p
1 views • 39 slides
NIMAS File Validation Using NIMAC Validation Wizard
Validate all files submitted to the NIMAC with the Validation Wizard before submission. The Validation Wizard ensures well-formed XML, correct image references, and more. Remember not to include the screen capture in the NIMAS file set. Download the Validation Wizard from the Resources page and foll
1 views • 17 slides
Global Multiplex Assay Market
The Global Multiplex Assay Market Size is expected to reach USD 7.1 Billion by 2032, at a CAGR of 7.3% during the forecast period 2022 to 2032.\n\n
1 views • 7 slides
Implementing Data Validation in National Accounts with Eurostat
Eurostat's ESA 2010 Validation Task Force has been instrumental in setting up validation checks, resolving recurrent validation problems, and enhancing the data validation process for National Accounts. The project involves participants from NSIs, Central Banks, and data users, focusing on refining
1 views • 29 slides
GOES-R Airborne Science Validation Field Campaign Overview
The GOES-R field campaign aimed to validate ABI and GLM products post-launch by coordinating the high-altitude NASA ER-2 aircraft with ground-based reference data over various Earth targets from March 21 to May 17, 2017. The primary objective was the independent validation of ABI spectral radiance o
1 views • 11 slides
Understanding Cross-Validation in Machine Learning
Cross-validation is a crucial technique in machine learning used to evaluate model performance. It involves dividing data into training and validation sets to prevent overfitting and assess predictive accuracy. Mean Squared Error (MSE) and Root Mean Squared Error (RMSE) quantify prediction accuracy,
0 views • 19 slides
CEOS LPV Fire Disturbance Products Overview
CEOS LPV Fire Disturbance products play a crucial role in monitoring and validating active fire and burned area datasets. The current status highlights the validation protocols in place for reference data generation, product inter-comparisons, and the need for validation information for multiple use
0 views • 9 slides
Snow Cover Validation Workshop 2013 Overview
Snow Cover Validation Workshop in 2013 focused on validating fractional snow cover data from November 1, 2012, to May 31, 2013. The workshop highlighted validation processes, tool statuses, product examples, algorithm enhancements, and post-launch activities. Key findings from granules demonstrated
0 views • 17 slides
Machine Learning Techniques: K-Nearest Neighbour, K-fold Cross Validation, and K-Means Clustering
This lecture covers important machine learning techniques such as K-Nearest Neighbour, K-fold Cross Validation, and K-Means Clustering. It delves into the concepts of Nearest Neighbour method, distance measures, similarity measures, dataset classification using the Iris dataset, and practical applic
1 views • 14 slides
Detection of Mutations in EGFR in Circulating Lung Cancer Cells: Study on SARMS Assay and CTC-Chip
This study by Shyamala Maherswaran, Ph.D., and team focuses on characterizing mutations in EGFR in circulating tumor cells using SARMS assay and CTC-chip. The research investigates the effectiveness of these non-invasive methods in analyzing tumors and explores the role of the T790M mutation in resp
0 views • 12 slides
Enhancing NIMAS File Validation with New Validation Wizard
The NIMAC introduced a new validation wizard in early 2022 to streamline the validation process for publishers and vendors. This tool provides advanced file examination, including feedback on OPF metadata and XML tagging errors. Learn how to use the tool efficiently for successful validation output,
0 views • 13 slides
Facilities Information Management System (FIMS) Data Validation Process
This document outlines the validation process for the Facilities Information Management System (FIMS), including objectives, validation team introductions, validation process steps, and the schedule for validation activities. It covers elements such as gauging the robustness of FIMS data, ensuring d
0 views • 18 slides
Protein Isolation Techniques: Salting In and Salting Out
Proteins exhibit varying solubility based on salt concentrations, with salting in increasing solubility and salting out causing precipitation. This practical involves isolating lactate dehydrogenase enzyme using salting in, salting out, and dialysis techniques, as well as assessing purity through ac
0 views • 19 slides
ESA Validation Approach & Fiducial Reference Measurements Overview
The validation approach outlined by ESA focuses on providing reliable products with documented error bars and enhancing algorithm and sensor quality. A validation program consists of various activities, including validation against precise reference measurements, in-situ validation, inter-satellite
0 views • 8 slides
Staff Data Validation Training 2023-2024 Overview
Learn about the staff data validation process for the 2023-2024 school year conducted by the Oregon Department of Education. Understand the importance of reviewing and verifying staff information for publication in various reports and profiles. Mark your calendar for key validation dates and explore
0 views • 49 slides
Understanding ASP.NET Validators and Validation Controls
ASP.NET validation controls play a crucial role in ensuring that user input data is valid and secure. They help prevent the storage of useless or contradictory data by validating input fields. Key validation controls include RequiredFieldValidator, RangeValidator, CompareValidator, RegularExpression
0 views • 37 slides
Understanding Validation Controls in ASP.NET for Secure Data Input
Explore the significance of validation controls in ASP.NET to prevent user input errors and ensure data security. Learn about the types of validators, server-side vs. client-side validation, key properties, and best practices for implementing secure data validation in your web applications.
0 views • 13 slides
Assay of Hydrogen Peroxide (H2O2) - Experimental Study
Hydrogen peroxide (H2O2) is a weak acid with both oxidant and reductant properties. It is an unstable compound that decomposes into oxygen and water. This experiment involves titrating hydrogen peroxide against a standard oxidizing agent solution to determine its concentration. The physical and chem
0 views • 9 slides
Evolution of Serials Validation Processes at OhioLINK
The evolution of serials validation processes at OhioLINK over the years, from the establishment of high-density storage facilities to the development and refinement of validation policies. The transition from issue-level to volume-level validation, evaluation of efficiencies, and the impact on staf
0 views • 8 slides
Understanding Validation in Authentic Assessment Items
Validation in authentic assessment items is crucial for ensuring the quality and effectiveness of assessment tools. It involves a thorough review process to confirm that the test items are valid, reliable, and produce authentic evidence for making sound judgments. Various aspects of validation, incl
0 views • 29 slides
SAGE III Validation Update: Mission Planning, Partnerships, and Criteria
Carrie Roller, the SAGE III Scientist/Engineer, provides an update on validation and mission planning, including partnerships with NDACC lidar and sonde sites, weekly coordination of SAGE III events with in-situ data, and the criteria for validation using in-situ data such as stratospheric ozone, ae
0 views • 13 slides
Overview of Sea Ice Characterization and Validation Process
Sea ice characterization and validation process for cryosphere products involve three stages of validation maturity. It includes evaluating algorithm performance, analyzing required inputs, conducting quality flag analysis, and documenting error budget. The team members consist of experts from vario
0 views • 38 slides
Nonparametric Tolerance Interval Approach to Immunogenicity Assay Cut Point Determination
This content discusses the importance of determining the cut point for immunogenicity assays to detect anti-drug antibodies (ADA) in large-molecule therapeutics. It highlights the need for well-developed and validated assays to ensure drug efficacy and safety. The approach involves multiple-tiered v
0 views • 26 slides
Insights from CCMC-Led Model Validation Challenges & International Coordination
Explore lessons learned from CCMC-led community-wide model validation challenges, emphasizing the importance of international coordination for monitoring and validation activities in the space weather domain. Discover key elements of model validation, community-wide metrics studies, and operational
0 views • 10 slides
Evolution of vCloud Air ISV Program in 2015
vCloud Air ISV Program underwent significant changes in 2015 with the introduction of automated validation, hybrid certification, streamlined processes, and enhanced technical engagement with VMware. ISV partners can now easily integrate with vCloud Air through the Developer Center, ensuring compati
0 views • 6 slides
Updates on GOES-R Aerosol Optical Depth Validation Activities
Generated proxy data algorithm enhancements for post-launch validation activities were discussed at the GOES-R AWG 2nd Validation Workshop in January 2014. The use of MODIS reflectances as a proxy for ABI to retrieve Aerosol Optical Depth (AOD) was emphasized, along with the importance of AERONET gr
0 views • 25 slides
Analyzing Ibuprofen Tablets: Experiment, Properties, and Testing
Ibuprofen, a non-steroidal anti-inflammatory drug, is examined through experiment, physiochemical properties, dosage forms, synthesis, and assay testing. Its identification tests include melting point, UV absorption, IR spectrum, and TLC analysis. The assay test method involves weighing and processi
0 views • 22 slides
Assay of NaOH Solution - Lab Procedure for Determining Sodium Hydroxide Content
This essay discusses the assay of NaOH solution, focusing on the two-step titration process involving NaOH and HCl for determining the concentration of NaOH. Detailed procedures, chemical factors, and calculations for both titration steps are explained in depth. The analysis also includes the ration
0 views • 10 slides
Statistical Tools for Method Validation in USP General Chapter 1210
In the USP General Chapter 1210, Statistical Tools for Method Validation are outlined, serving as a companion to the validation of Compendial Procedures. The chapter covers important topics like Accuracy, Precision, Linearity, LOD, LOQ, and range. It emphasizes statistical tools such as TOST, statis
0 views • 22 slides
Development of NSF Process for Validation and Acceptance of EVMS: Insights and Recommendations
NSF is enhancing guidelines for Earned Value Management Systems (EVMS) to evaluate construction project status. Insights suggest obtaining certification and validation for maintaining acceptable EVM systems, important for project oversight. Recommendations highlight the need for decisive action to e
0 views • 16 slides