MTT Cell Proliferation Assay: Protocol and Applications
The MTT cell proliferation assay is a widely used technique to measure cell viability, proliferation, and cytotoxicity in research. This colorimetric assay involves the conversion of MTT reagent to formazan dye by live cells, resulting in a purple color change. This comprehensive guide provides step-by-step instructions for conducting the assay, equipment and materials required, applications such as cell proliferation assays and cytotoxicity analysis, and the significance of MTT assay in assessing cell viability and growth responses to different stimuli.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
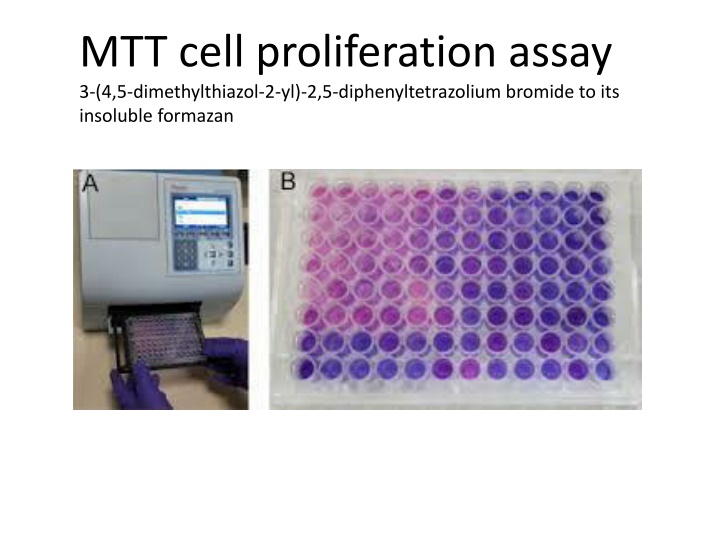
MTT cell proliferation assay 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide to its insoluble formazan
Applications: Cell proliferation assays Cytotoxicity analysis Apoptosis screening
Equipment and Materials Required Microtiter plate reader with 650- and 570-nm filters Microtiter plate (flat-bottomed) Inverted microscope Sterile tubes (5 mL) Multi-channel pipette Serological pipettes 37 C incubator Sterile pipette tips Laminar flow hood
Step Action 1 Harvest suspension cells by centrifugation. Adherent cells should be released from their substrate by trypsinization or scraping. 2 Resuspend cells at 1 x 106 per mL. 3 Prepare serial dilutions of cells in culture medium from 1 x 106 to 1 x 103 cells per mL. 4 Plate out, in triplicate, 100 L of the dilutions into wells of a microtiter plate. 5 Include three control wells of medium alone to provide the blanks for absorbance readings 6 Incubate the cells under conditions appropriate for the cell line for 6 to 48 hours (to recover from handling). The time required will vary but 12 hours to overnight is sufficient for most cell types. 7- Add 10 L of MTT Reagent to each well, including controls. 8- Return plate to cell culture incubator for 2 to 4 hours.
9- Periodically view the cells under an inverted microscope for presence of intracellular punctate purple precipitate. 10- When the purple precipitate is clearly visible under the microscope add 100 L DMSO Dimethyl sulfoxide into each well to dissolve the formazan by pipetting up and down several times gently; do not shake, including controls. 11- Leave plate with cover in the dark for 2 to 4 hours or overnight at room temperature. 12- Remove plate cover and measure the absorbance in each well, including the blanks, at 570 nm in a microtiter plate reader. [Absorbances can be read with any filter in the wavelength range of 550 - 600 nm. The reference wavelength should be higher than 650 nm. The blanks should give values close to zero (+/- 0.1).] 13- If the readings are low return the plate to the dark for longer incubation. 14- Determine the average values from triplicate readings and subtract the average value for the blank. Plot absorbance against number of cells/mL. The number of cells to use in your assay should lie within the linear portion of the plot and yield an absorbance of 0.75 - 1.25.
MTT assay The MTT assay and the MTS assay are colorimetric assays for measuring the activity of enzymes that reduce MTT or close dyes (XTT, MTS, WSTs) to formazan dyes, giving a purple color The main application allows to assess the viability (cell counting) and the proliferation of cells (cell culture assays) It can also be used to determine cytotoxicity of potential medicinal agents and toxic materials, since those agents would stimulate or inhibit cell viability and growth
The MTT Cell Proliferation and Viability Assay is a safe, sensitive, in vitro assay for the measurement of cell proliferation or, when metabolic events lead to apoptosis or necrosis, reduction in cell viability. Cells are cultured in flat-bottomed, 96-well tissue culture plates. The cells are treated as per experimental design and incubation times are optimized for each cell type and system. The tetrazolium compound MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5- diphenyltetrazolium bromide) is added to the wells and the cells are incubated. MTT is reduced by metabolically active cells to insoluble purple formazan dye crystals by mitochondrial enzymes associated with metabolic activity. The reduction of MTT is primarily due to glycolytic activity within the cell and is dependent upon the presence of NADH and NADPH
Synthesis There various synthetic methods for the synthesis of formazans. The reaction of diazonium compounds with aldehyde hydrazones is one of the most common procedures to produce formazans. Hydrazones, which are electron-rich compounds, react with diazonium salts either at a nitrogen or a carbon atom to produce formazans. Diazonium salts couple to the amine nitrogen in the hydrazone with displacement of a hydrogen to give the intermediate, which then rearranges to the formazan. Another form to synthesize formazans is by the reaction of active methylene compounds with diazonium salts. Diazonium salts add to active methylene compounds to form an intermediate azo compound, followed by the addition of a second diazonium salt (under more alkaline conditions), yielding tetrazene, which then forms a 3-substituted formazan. Formazans can also be produced by the oxidation of the corresponding hydrazidines, usually prepared via reaction of hydrazonyl halides with the appropriate hydrazine derivatives. For example, ethyl formate or orthoformate reacts with two equivalents of phenylhydrazine to yield 1,5-diphenylformazan, under acidic conditions. Under basic conditions, ethyl nitrate reacts at the methylene position to yield 3- methyl-1,5-diphenylformazan, which can also be obtained from the reaction of phenylazoethane with isoamyl nitrite. Additionally, formazans can be obtained by the decomposition of substituted tetrazolium salts either photochemically or under the influence of ascorbic acid in an alkaline medium.[11]