Understanding Valence Electrons in Chemistry
Explore the concept of valence electrons with definitions, diagrams, and explanations of how electrons are located in the electron cloud. Learn about the importance of valence electrons in determining an element's reactivity and stability. Discover how atoms gain or lose electrons to achieve a full outer shell and why elements with specific numbers of valence electrons exhibit varying chemical reactivity.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
8th grade Read p.51-53 Do questions 1-2, on page 51,Do definitions p. 51 and Do question 3 a and b p. 53 8th (please ask students what chapter we are on so everyone will be on the same page) History: Flashcards for definitions and identify for chapter 5.2 and 5.3, when done outline for chapter 5.4 and flashcards for 5.4. Whatever is not done is to be done as HWK. Valence Electrons
Where are Electrons located? In the Electron Cloud
How many Levels are in the Electron Cloud? Three
How many Electrons fit in the 1st/2nd/3rd Energy Levels? 1st Energy Level = 2 e 2ndEnergy Level = 8 e 3rdEnergy Level = 8 e
Valence Electrons These are the electrons on the atom s OUTERMOST or LAST Energy Level/shell
1. What element is pictured? 2. How many protons? + + + 3. How many electrons? 4. How many neutrons? 5. What is the atomic number? 6. What is the atomic mass? 7. How many valence electrons?
+ + + + + + + + + + +
+ + + + + + + + + + + A. B. + + + + + + + C.
Valence Electrons Valence Electrons are used to determine an element s chemical reactivity (how easily it will react or combine or bond with other elements)
Valence Electrons ALL atoms want to have a FULL outermost shell Atoms with a full outermost shell are the MOST stable, so they have a very low chemical reactivity. The Noble Gases Group 18 all have a full outer shell
Valence Electrons Atoms will gain or lose electrons to get a full outer shell. It is a lot easier to lose or gain only 1 electron, so those elements with only 1 valence electron have a very high chemical reactivity.
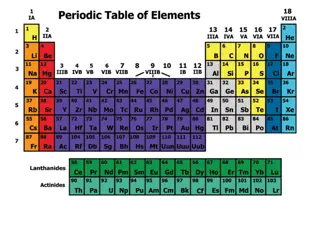
Valence Electrons Elements in group 1 only have 1 valence electron, so they must lose 1 electron to have a full outer shell. This makes all elements in group 1 highly reactive. Elements in group 17 have 7 valence electron, so they must gain 1 electron to have a full outer shell. This makes all elements in group 17 highly reactive.
Valence Electrons All other elements with 2, 3, 4, 5, or 6 valence electrons have a moderate chemical reactivity. It is easier to gain/lose 2 electrons than to lose 3 electrons, so elements with 2 valence electrons are more reactive than those that must gain/lose 3 electrons.