Understanding the Periodic Table: Mendeleev's Discovery and the Role of Valence Electrons
Russian chemist Dmitri Mendeleev's groundbreaking work in 1869 led to the creation of the periodic table, showcasing a pattern in the arrangement of elements by increasing atomic mass. Discover how Mendeleev's table evolved, the significance of Henry Moseley's contribution in 1914, and the importance of valence electrons in determining the properties of elements within the table's groups.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
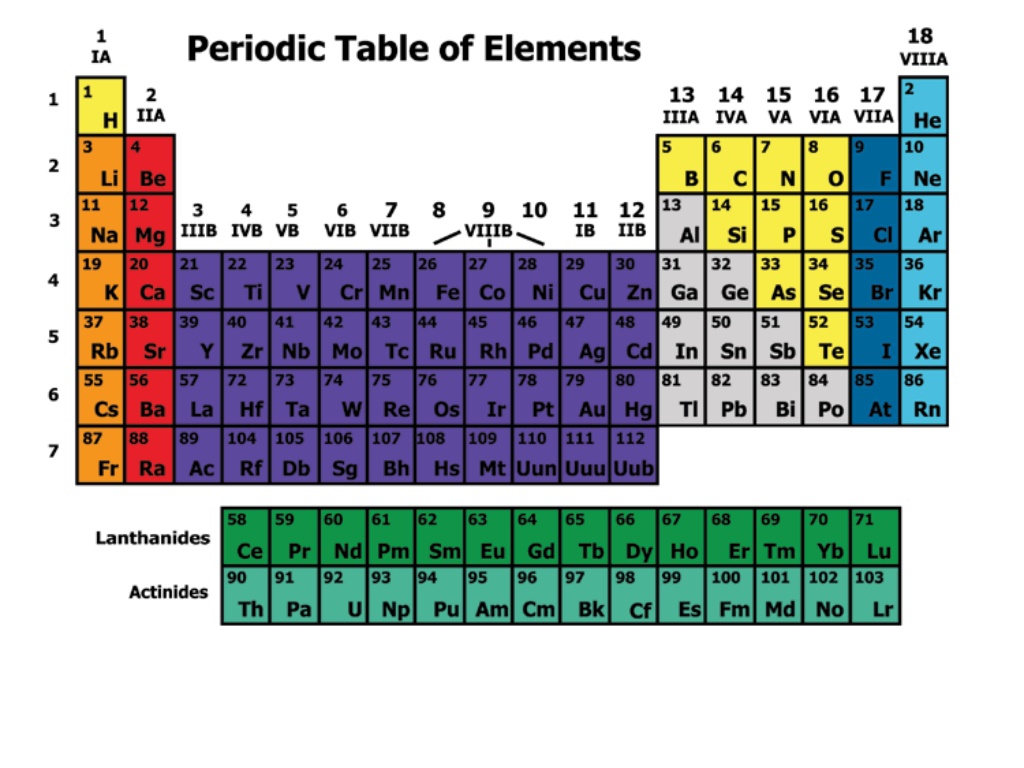
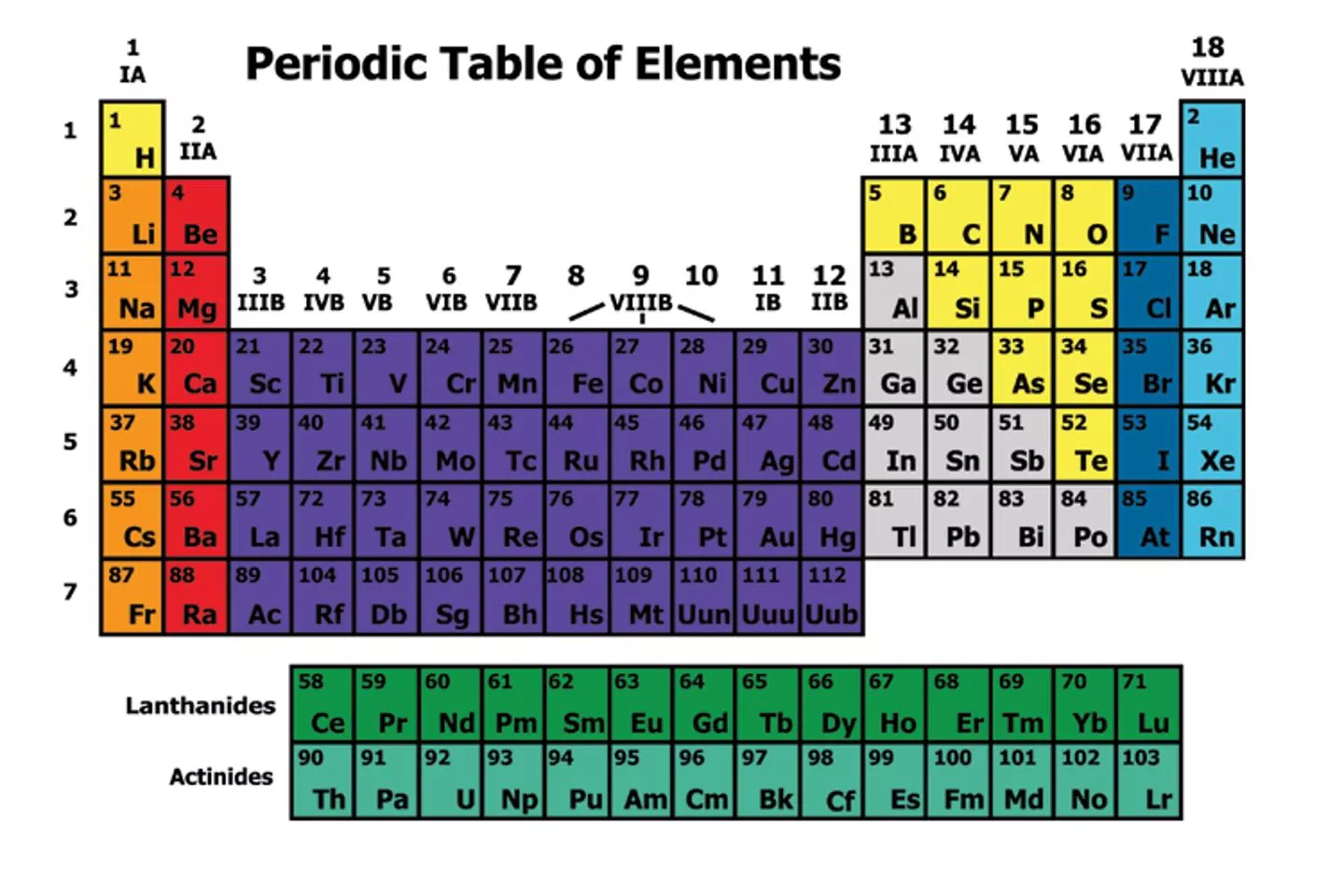
Discovering a Pattern In 1869, Russian chemist Dmitri Mendeleev arranged the elements in order of increasing atomic mass.
His Periodic table showed that Elements with similar properties occurred in a repeating pattern There were gaps in its pattern He could predict the properties of the missing elements By 1886, all of the gaps had been filled and Mendeleev s predictions were right.
Changing the Arrangement A few elements properties did not fit in the pattern of Mendeleev s table. 1914: British scientist Henry Moseley found the atomic number of atoms. Elements arranged by atomic number fit the pattern in Mendeleev s table.
Elements are arranged: Vertically into Groups Horizontally Into Periods
Each group has distinct properties The periodic Table is divided into several groups based on the properties of different atoms.
If you looked at one atom of every element in a group you would see
Each atom has the same number of electronsin it s outermost shell. An example
The group 2 atoms all have 2 electrons in their outer shells Be (Beryllium) Atom Mg (Magnesium) Atom
Valence electrons Definition: the number of electrons in the outer shell of an element Electrons held most loosely an outer shell is considered complete when it contains 8 electrons Each element has specific number from 1 to 8 effects the way an atom bonds Period ends when valence = 8 determines many properties of the element elements within a group usually have similar properties.
One way to represent atoms and molecules is to use electron-dot diagrams. An electron-dot diagram shows only the valence electrons in an atom.
If you looked at an atom from each element in a period you would see
Each atom has the same number of electron holding shells. An example
The period 4 atoms each have 4 electron containing shells 4th Shell K (Potassium) Kr (Krypton) Atom Atom Fe (Iron) Atom
The Periodic Table and Classes of Elements Elements are classified as metals, nonmetals, and metalloids based on number of electrons in outer shell The zigzag line on the periodic table can help you recognize which elements belong in which category.
Metals found to the left of the zigzag line have few electrons in their outer energy level. shiny, ductile, malleable, and are good conductors of electric current and thermal energy.
Metalloids border the zigzag line have about half of a complete set of electrons in their outer energy level. Metalloids have some properties of metals and some properties of nonmetals. Metalloids are also called semiconductors.
Nonmetals to the right of the zigzag line have an almost complete set of electrons in their outer energy level. not shiny, ductile, or malleable, and poor conductors of electric current and thermal energy.
Group 1: Alkali Metals Alkali metals properties: metals 1 electron in the outer level very reactive softness, color of silver, shininess, low density
Alkali Metals Soft, silvery colored metals Very reactive!!!
Alkali Metals reacting with water: Li (Lithium) Na (Sodium) K (Potassium) Rb (Rubidium) Cs (Cesium) What would you expect from Francium?!?!
Group 2: Alkaline-Earth Metals Alkaline-earth metals properties: metals 2 electrons in the outer level very reactive, but less reactive than alkali metals color of silver, higher densities than alkali metals
Alkaline Earth Metals Silvery-White Metals Fairly reactive Many are found in rocks in the earth s crust
Group 312: Transition Metals Properties of Transition Metals vary widely but include: metals 1 or 2 electrons in the outer level less reactive than alkaline-earth metals shininess, good conductors of electric current and thermal energy
Transition Metals Most are good Conductors of electricity Malleable (easily bent/hammered into wires or sheets)
How many things can you think of that have Transition Metals in them?
Metalloids lie on either side of these stairsteps They share properties with both metals and non-metals Si (Silicon) and Ge (Germanium) are very important semi-conductors
Nonmetals Brittle Do not conduct electricity
Group 13: Boron Group Group 13 properties: one metalloid and five metals 3 electrons in the outer level reactive solids at room temperature
Group 14: Carbon Group Group 14 properties: one nonmetal, two metalloids, and two metals 4 electrons in the outer level reactivity varies among the elements solids at room temperature
Group 15: Nitrogen Group Group 15 properties: two nonmetals, two metalloids, and two metals 5 electrons in the outer level reactivity varies among the elements solids at room temperature (except for nitrogen, which is a gas)
Group 16: Oxygen Group Group 16 properties: group contains three nonmetals, one metalloids, and one metal 6 electrons in the outer level reactive solids at room temperature (except for oxygen, which is a gas)
Halogens Most are Poisonous Fairly reactive
Section 2 Grouping the Elements Chapter 12 Group 17: Halogens Halogens are the elements in Group 17. Group 17 properties: group contains nonmetals 7 electrons in the outer level very reactive poor conductors of electric current, never in uncombined form in nature
Chlorine Gas was used as a chemical weapon during World War I. It was used by the Nazis in World War II.
Section 2 Grouping the Elements Chapter 12 Group 18: Noble Gases Noble gases are the elements in Group 18. Group 18 properties: group contains nonmetals 8 electrons in the outer level (except helium, which has 2) unreactive colorless, odorless gases at room temperature
Noble Gases Unreactive Gases at room temperature
Jellyfish lamps made with noble gases artist- Eric Ehlenberger
Colors Noble Gases produce in lamp tubes: Ne (Neon): orange-red Hg (Mercury): light blue Ar (Argon): pale lavender He (Helium): pale peach Kr (Krypton):pale silver Xe (Xenon): pale, deep blue
Group 312: Transition Metals, continued Lanthanides and Actinides Some transition metals from Periods 6 and 7 appear in two rows at the bottom of the periodic table. Elements in the first row are called lanthanides and elements in the second row are called actinides.
Lanthanide Series Actinide Series
Hydrogen The properties of hydrogen do not match the properties of any single group, so hydrogen is set apart. a nonmetal 1 electron in the outer level reactive colorless, odorless gas at room temperature, low density
Section 2 The Atom Chapter 11 Isotopes Isotopes are atoms that have the same number of protons but have different numbers of neutrons.
Section 2 The Atom Chapter 11 Isotopes, continued Properties of Isotopes An unstable atom is an atom with a nucleus that will change over time. This type of isotope is radioactive. Telling Isotopes Apart You can identify each isotope of an element by its mass number. The mass number is the sum of the protons and neutrons in an atom.
Section 2 The Atom Chapter 11 Isotopes, continued
Naming Isotopes: write the name of the element followed by a hyphen and the mass number of the isotope. Example: Uranium-238 The atomic mass of an element is the weighted average of the masses of all the naturally occurring isotopes of that element.
Section 2 The Atom Chapter 11