Understanding Electron Theory and the Structure of Matter
The structure of matter is intricately tied to electron theory, where molecules are made up of atoms containing protons and electrons. An electrically neutral atom has an equal number of protons and electrons. A flow of electrons creates an electric current, which can be measured by an ammeter. Conductors and insulators play key roles in electrical conductivity. The effects of an electric current include thermal, chemical, and magnetic effects.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
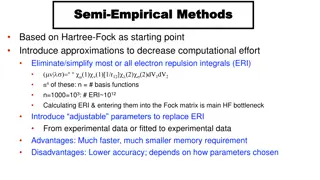
Electron theory Electron theory Structure of matter The smallest part of any material is called a molecule and is made up of one or more atoms. Basically, the atom is constructed of a central core containing protons surrounded by orbiting electrons. Protons are positively charged. Electrons are negatively charged. In an electrically neutral atom, the number of protons is equal to the number of electrons.
The simplest atom is hydrogen (1 proton and 1 electron). The heaviest atom is uranium (92 protons balanced by 92 electrons). The Helium atom has two protons and two electrons
Copper atom 29 protons and 29 electrons An electrically neutral atom has as many (+ve positive) protons as there are (-ve negative) electrons. The single electron in the outer orbit of the copper atom is only loosely attached to the atom.
Electric current random free electron movement If the material is connected across a battery, the positive plate (or terminal) attracts electrons, whilst the negative plate repels them. The battery provides a source of electromotive force (EMF). The resultant electron flow around the circuit is called an electric current.
Conductors and Insulators Insulators Conductors Silver Gold Copper Aluminium Mercury Steel Sea water Concrete Mercury Platinum Brass Iron Bronze Graphite Soiled water Lemon juice Rubber Glass Pure water Oil Air Diamond Dry wood Dry cotton Plastic Asphalt Fibreglass Dry paper Porcelain Ceramic Quartz
Effects of an electric current Thermal
Effects of an electric current Thermal Chemical
Effects of an electric current Effects of an electric current Thermal Chemical Magnetic
Join us at Aldershot College and learn how to become an electrician. The electrical industry is ever expanding and the demand for electricians are constant. Huge demand for Electricians in the commercial, industrial, and domestic sectors Electrical is one of the top trades in the industry.