Understanding Valence Electrons and Ionic Charges in Elemental Bonding
Valence electrons play a crucial role in the formation of ions as elements combine. Nonmetals gain electrons to become negatively charged ions, while metals lose electrons to become positively charged ions. This process leads to the creation of electrically attractive elements open for bonding. The exchange of electrons in elemental bonding results in complete outer energy levels and the formation of stable ions with distinct charges. Explore how elements in different groups behave in acquiring or losing electrons to achieve a balanced state in chemical bonding.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
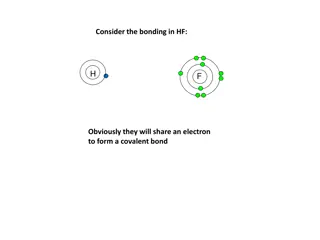
Valence electrons Valence electrons Valence electrons are those electrons that are lost or gained when elements combine.
Nonmetals will gain electrons when forming compounds. They become negatively charged because there will be more electrons than protons. A negative ion will be formed. 17P = +17 18E= -18 Cl 17P 18N 2 8 7 ionic charge: 1E= -1
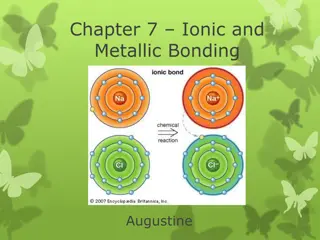
Metals will lose electrons when forming compounds. They become positively charged because there will be more protons than electrons. A positive ion is formed. Na 11P 12N 2 8 1 11P = +11 10E = -10 ionic charge: 1P = +1
Sodium loses one electron. Sodium now has 11 protons and 10 electrons, (+1). Chlorine gains one electron. Chlorine now has 17 protons and 18 electrons, (-1).
Metals will lose 1, 2, or 3 electrons in the last energy level to other elements. This will leave the last shell complete. Nonmetals will gain 1, 2, or 3 electrons onto their outside energy level from other elements. This will complete the last shell.
Valence electrons are electrons that are lost or gained when elements combine. Ions are elements that lose or gain an electron. They then become electrically charged, therefore electrically attractive and open for bonding!
Elements in Group 1 will lose 1 electrons and become positive (+1) ions. Elements in Group 2 will lose 2 electrons and become positive (+2) ions. Elements in Group 13 will lose 3 electrons and become positive (+3) ions. Elements in Group 14 can either lose or gain 4 electrons, either (+/-4) ions.
CARBON C4 C NEUTRAL ATOM 6P = +6 6E = -6 0 = 0 POSITIVE ATOM 6P = +6 2E = -2 4P = +4 NEGATIVE ATOM 6P = +6 10E = -10 4E = -4 6P 6N 2 4 6P 6N C 4 2 C 4 6P 6N 2 8
Elements in Group 15 will gain 3 electrons and become negative (-3) ions. Elements in Group 16 will gain 2 electrons and become negative (-2) ions. Elements in Group 17 will gain 1 electron and become negative (-1) ions.
Bohr Model Bohr models show every electrons on each energy level of an atom.
DOT DIAGRAMS DOT DIAGRAMS Dot diagrams show the electrons on the last energy level of an atom. Metals will have 1, 2, 3, or 4 electrons. Ex: Sodium Bohr Model 11P 12N 2 8 1 To write the Dot Diagram for this element you would write Na .
In nonmetals, these are the electrons that are on the last energy level. There will be *4, 5, 6, 7, or 8 electrons. Ex: Chlorine 17P 18N Bohr Model 2 8 7 To write the Dot Diagram for this element you would write . . : Cl :