Overcoming Misconceptions in Chemistry Education
Addressing misconceptions in general chemistry education, focusing on student knowledge from high school Math and Chemistry courses. Emphasis on student motivation, learning strategies, and common misconceptions in understanding scientific concepts. Strategies for enhancing comprehension, application, and retention in chemistry education are explored.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
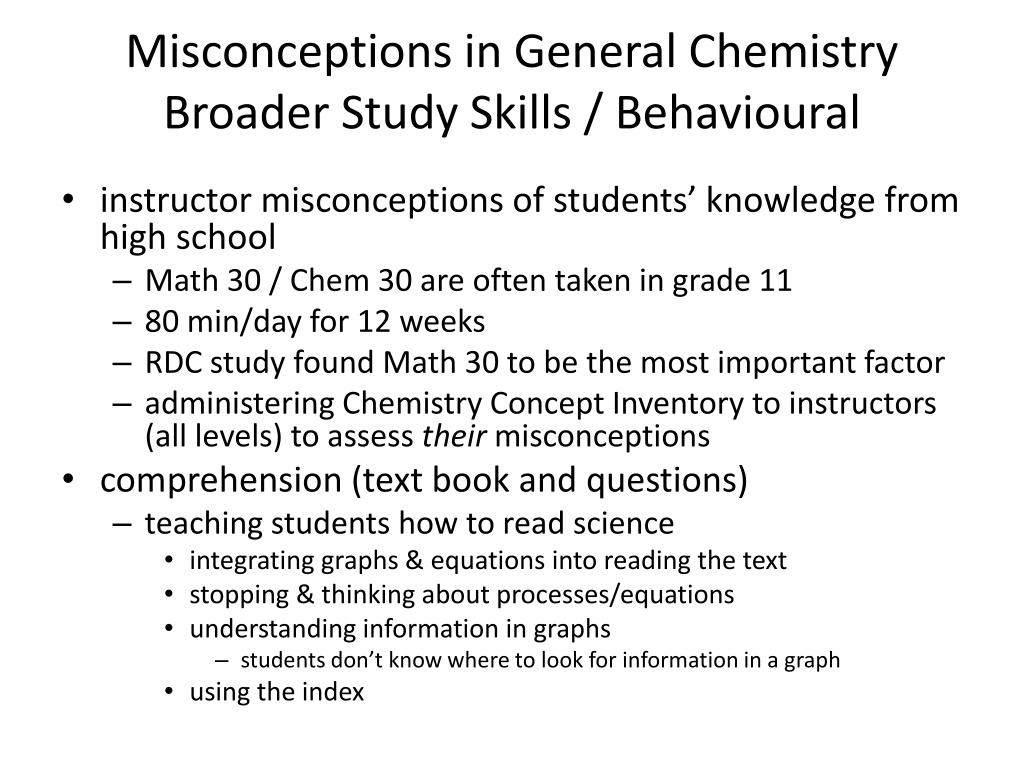
Misconceptions in General Chemistry Broader Study Skills / Behavioural instructor misconceptions of students knowledge from high school Math 30 / Chem 30 are often taken in grade 11 80 min/day for 12 weeks RDC study found Math 30 to be the most important factor administering Chemistry Concept Inventory to instructors (all levels) to assess their misconceptions comprehension (text book and questions) teaching students how to read science integrating graphs & equations into reading the text stopping & thinking about processes/equations understanding information in graphs students don t know where to look for information in a graph using the index
Broader Study Skills / Behavioural misconception about student motivation most of them are not like us! chemistry is not a priority in first year non-majors! student misconception about required work passed high school with rote memorization (e.g., studying for the 5 (or 7) question types used by AB Education in provincial test) not used to scheduling self-study time think they know it all (repetition from high school) challenging to get them to comprehension, application misconception about student learning: Students will learn what we teach no..mostly will only learn what we test assessment is the tail that wags the dog
Broader Study Skills / Behavioural student misconception of easy problems only in lecture watching the expert make no mistakes they can follow (but maybe not lead so well) student misconception: don t need to know vocabulary/jargon student misconception of separation of disciplines why is general chemistry more like physics? chemistry is a math course instructor misconception of how general chemistry should be taught (content phys chem heavy)?
General Misconceptions misconception of size/scale chemistry is unfathomable in magnitude getting across just HOW small an atom is Powers of 10 video/interactive website Stacey s comparison of 1 mol paperclips vs. money vs. water misconception that chemistry is bad inform about quantity (toxicity is in the dose) clearing up misconceptions: use break time or 5 min at beginning of last lecture every week chemistry in the news topic of interest related or unrelated to current lecture resources such as Joe Schwarcz books misconception that a proton exists as H+ or H3O+
Misconceptions about Scientific Method we don t communicate that we are teaching models, don t communicate scientific method science as the fountain of knowledge , misconception that science is about facts, wrong & right answers science is ongoing! it s happening right now! confused that something called a law can fail
Heat, Energy, Temperature Misconceptions breaking bonds produces energy biology: ATP -> ADP + energy heat, energy, temperature terms of general use, students have preconceptions hard to redefine precisely, in contrast to new terms compare: strong vs. concentrated (e.g., coffee) heat is misconceived as a noun by both Teaching aid: Youtube Eureka #21 Heat vs. T
Physical Transitions Misconceptions misconceptions/concepts start early AB 5thgrade is about half of first year without math difference between melting and dissolution, liquid and aqueous instructor misconception: students have particle view of matter matter is bulk to students visualization: initiative at King s see kcvs.ca for examples Odyssey (~$75) visualization software e.g. linear vs. bent H2O - properties come from structure using visualizations: test what you teach! suggestion: project coloured images on screen during exam Teaching Aids: Children s Ideas and the Learning of Science by Rosalind Driver et al. Talanquer, V. et al. A2: Element or Compound J. Chem. Ed. 2007, 84, 880.
Stoichiometry and limiting reagents how to teach this best? sandwich example (bread, cheese, meat) macaroni/choc chip cookie example (how much of each ingredient) misconception: students transfer the process (compartmentalization issue, as in K and other areas) again, need to get to particle view of matter students don t make connections between letters/symbols and particles (maybe we don t either until we teach) Teaching Aids: See publications by Miles Pickering and Mary Nockley(?) George M. Bodner, I have found you an argument: The conceptual knowledge of beginning chemistry graduate students J. Chem. Ed. 1991, 68, 385.
Equilibrium Misconceptions like heat/T, there is a meaning attached already misconception: equilibrium means equal mass (not rate) Stacey s demo: 2 fish tanks (one empty, one full) 2 beakers scooping water from either side to the other ask students to predict, when equilibrium is reached equilibrium problems ALL look different to some students
Electrochemistry Misconceptions where do electrons come from? (electron transfer) concept of potential necessity in 1st year? helpful doodles to show electrons transferring in or out of electrodes, corrosion and plating at electrodes etc. Literature tip: T. J. Greenbowe & M. J. Sanger
Significant Figures misconception/disagreement regarding importance order of magnitude vs. actual sig. figs. includes ability to evaluate an answer for validity our obligation goes beyond just teaching chemistry (but how far?) introduce concept in context in labs (+ use in lecture) student misconception of meaning/origin math rules come from physical reality of measurement introduced too early in text book, needs more context (lab)
Lab Lecture Connectivity students expect a link whose misconception? issue of integrating early lectures with lab effort on side of lecturer labs as concept reinforcement misconception about how we learn? learning by doing: discovery labs closer to scientific method / development of models possible to pick up student data in lecture (.:relevance)