Understanding the Inductive and Electromeric Effects in Organic Chemistry
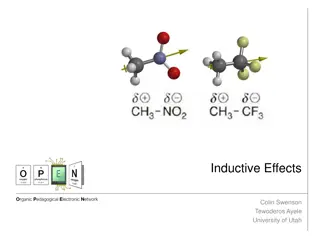
The inductive effect in organic chemistry is the polarization of a bond due to electron-donating or withdrawing effects of adjacent groups, leading to a degree of polarity in the bond. This effect is distance-dependent and can be either electron-withdrawing or electron-releasing. On the other hand, the electromeric effect refers to the polarity produced in a multiple bonded compound when approached by a reagent, resulting in the transfer of electrons between atoms. Understanding these effects is crucial in studying chemical reactivity and molecular behavior.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
INDUCTIVE EFFECT & ELECTROMERIC EFFECT PREPARED BY MR. NARAYAN P. PAWAR (PHARMACEUTICAL CHEMISTRY)
Inductive effect Definition:- The polarization of bond due to electron withdrawing or electron donating effect of adjacent groups or atoms is called inductive effect. Or It is defined as permanent displacement of shared electron pair in carbon chain towards more electronegative atoms or group is called inductive effect. CH3 Cl CH3 Cl CH3 -Cl Methyl chloride
It involves electrons. The electrons which forms a covalent bond are shared equally between the two atoms. The inductive effect (I effect) refers to the polarity produced in a molecule as a result of higher electronegativity of one atom compared to another. This is because different atoms have different electronegativity values. This introduce a certain degree of polarity in the bond. The more electronegative atom acquires a small negative charge (- ). The less electronegative atom acquires a small positive charge (+ ).
H2 C 2 H2 C 1 H3C Cl 3 The inductive effect of C3 upon C2 is significantly less than the effect of the chlorine atom on C1. It is distance dependent. There are two types: 1) Electron-withdrawing/ attracting inductive effect (-I Effect) Those atoms or groups which withdraw electrons away from another atoms are said to be I effect. e.g. F, -Cl, -Br, -I, -OH, -NH2, .etc.
2) Electron- releasing/ losing inductive effect (+I effect) Those atoms or groups which lose electrons towards another atom are said to be +I effect. e.g. (CH3)3C- , (CH3)2CH- , CH3-CH2- , CH3- - Shifting of electrons in covalent bond from low electronegative atom to high electronegative atom. - Always sigma ( ) electrons are displaced (only occur in single boned). - it is permanent effect.
Electromeric effect:- Definition:- The electromeric effect (E effect) refers to the polarity produced in a multiple bonded compound as it is approached by a reagent. when a double or a triple bond is exposed to an attacking by a reagent, the two electrons which form the bond are completely transferred to one atom or the other. a t t a c k i n g r e a g e n t H 2C C H H 2C C H 2 e.g. 2 e t h e n e 2a t t a r e c k i n g a g e n t H 2 C C H 2 H 2 C C e t h e n e H
O O attacking reagent C C H3C CH3 H3C CH3 Acetone a t t a c k i n g r e a g e n t H C H 3C C H C H H 3C C H 2 2 p r o p e n e
Their are two types of electromeric effect: 1) + E effect : If electron pair shifted towards the site where attacking reagent to be attached. e.g. + H C H H 3C C H C H H 3C C H 2 3 a t t a c k i n g r e a g e n t p r o p e n e O O H +H C H 3 C C H C H 3 C C H 3 3 A c e t o n e a t t a c k i n g r e a g e n t
2) E effect : If electron pair shifted away from the site where attacking reagent to be attached. e.g. H C O H + H 3C C H C H H 3C C H 2 2 a t t a c k i n g r e a g e n t O H p r o p e n e O O +C N C H 3C C H C H 3 C C H 3 3 a t t a c k i n g r e a g e n t A c e t o n e C N
Use of electromeric effect: Use of electromeric effect: C C H H C C N N H H H H O O H H + + C C 1) 1) H H C C N N 3 3 3 3 O O H H a a c c e e t o t o n n i t r i t r i l e i l e a a c c e e t i m t i m i d i d H C C H i c i c a a c c i d i d H 3 C + H C N C H H 3 C C H 3 2 2) 2) C N p r o p - 1 - e n e i s o b u t y r o n i t r i l e h y d r o g e n c y a n i d e O H O C C H 3 C H 3 +H C N C C H 3 C H 3 3) 3) C N e t h y l p r o p a n e n i t r i l e p r o p a n - 2 - o n e h y d r o g e n c y a n i d e 2 - h y d r o x y - 2 - m
- Temporary effect, observed only in reaction. - It involves the electron. - Displacement of pi ( ) electron only. - Only occur in presence of attacking reagent.