Understanding Atomic Mass and Isotopes in Atoms
Explore the concept of atomic mass in atoms, learn to compute atomic mass and mass number, identify isotopes, and calculate the number of neutrons in an atom. Understand the significance of the atomic number and mass number in determining the characteristics of elements.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
SECTION 2: MASSES OF ATOMS Chapter 17: Properties of Atoms
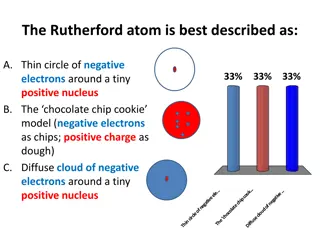
WARM-UP: Which of the following can change within an element and not change the identity of that element? Proton Neutron Electron
LEARNING GOALS Compute the atomic mass and mass number of an atom. Identify the components of isotopes. Interpret the average atomic mass of an element.
ATOMIC MASS The nucleus contains most of the mass of the atom. Protons and neutrons are far more massive than electrons The mass of a proton and a neutron is almost 1 atomic mass unit (amu)
ATOMIC MASS Different elements are distinguished by the number of protons that their atoms have Every carbon atom has six protons Every oxygen atom has eight protons Atomic Number: the number of protons in an atom
ATOMIC MASS Mass Number: the sum of the number of protons and the number of neutrons in a single atom If you know the mass number and the atomic number, you can calculate the number of neutrons # of neutrons = mass number - atomic number
ATOMIC MASS Example: An atom of potassium (K) has a mass number of 40. How many neutrons does it have?
ISOTOPES Not all atoms of an element have the same number of neutrons. Isotopes: atoms of the same element that have different numbers of neutrons
ISOTOPES Suppose you have a sample of boron (B): Naturally occurring atoms of boron have mass numbers of 10 or 11. How many neutrons are in each isotope of boron?
ISOTOPES Because most elements have more than one isotope, each element has an average atomic mass. Average atomic mass: the weighted average of mass of the mixture of its isotopes The average atomic mass for each element is listed in the periodic table!
ISOTOPES Four out of every five atoms of boron are boron-11 and one out of every five are boron-10. 11 11 11 11 10 4/5(11 amu) + 1/5(10 amu) = 10.8 amu
CHECK-IN: What is the mass number of an isotope of neon (Ne) that has 11 neutrons? What is the average atomic mass of neon?