Understanding Models of the Atom: Nucleus, Protons, Electrons
Dive into the fundamental concepts of atoms, exploring their structure with a focus on the nucleus, protons, electrons, and neutrons. Discover how the periodic table reveals crucial information about elements, such as atomic number and mass number. Explore the evolution of atom models and the necessity of using models to understand the unseen world of atoms.
Download Presentation
Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
Presentation Transcript
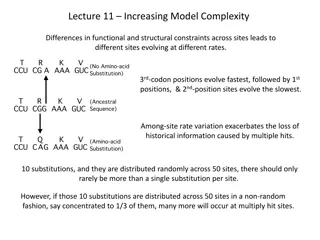
STARTER Page 6 of your metals, ions and the periodic table workbook. What are the two main areas in an atom? What do we find in them? What do the two numbers on the periodic table tell us about an element?
STARTER What are the two main areas in an atom? - The nucleus and the shells. What do we find in them? + + We find protons and neutrons in the nucleus We find electrons in the shells -
STARTER What do the numbers on the periodic table tell us about an element? The atomic (or proton) number tells us the number of protons in the nucleus. In a neutral atom it also tells us the number of electrons. atomic (proton) number
STARTER What do the numbers on the periodic table tell us about an element? mass number The mass number is the SUM of the protons and neutrons To calculate neutrons, we subtract the proton number from the mass number neutrons = mass number proton number
STARTER What do the numbers on the periodic table tell us about an element? mass number protons = atomic (proton) number electrons = atomic (proton) number neutrons = mass number proton number atomic (proton) number
MODELS IN SCIENCE Take 2 minutes to discuss with a partner - 1. What do we use models for in everyday life? + 1. Why might we need a model to think about atoms? + - This is a model
MODELS IN SCIENCE Take 2 minutes to discuss with a partner - Models let us imagine and describe things that are too small, too big or too dangerous for us to see + + We need a model to describe atoms because they are too small for us to see! -
MODELS IN SCIENCE How we imagine and describe atoms has changed a lot over time
MODELS IN SCIENCE Show the video!
Sketch this scientists model of the atom here: Name: Democritus Date of theory: 400 BCE What was the smallest quantity of matter according to this scientist? Atomos this is where we get the modern word ATOM from!
Sketch this scientists model of the atom here: Name: John Dalton Date of theory: 1800s Whose idea did this scientist revive? Democritus How did this scientist describe atoms? Solid spheres What did this scientist theorise about compounds? Compounds are made of different types of atom joined together
Sketch this scientists model of the atom here: Name: J J Thompson Date of discovery: 1897 What did this scientist discover? The Electron What is the name of this scientist s model of the atom? The Plum Pudding model What did this scientist disprove about Dalton s theory? Dalton said that atoms could not be broken into smaller parts, J J Thompson proved this wrong.
Sketch this scientists model of the atom here: Name: Ernest Rutherford Date of discovery: 1911 What did this scientist discover? The existence of the nucleus What is the name of this scientist s model of the atom? The planetary model What experiment did this scientist use to develop his theory? The gold foil experiment
Sketch the experiment here: Names: Geiger and Marsden Date of experiment: 1905 Who were these scientists working for? Ernest Rutherford What experiment did they carry out? The gold foil experiment Which model of the atom did their work disprove? The plum pudding model How did their experiment disprove it? It showed that positive charge in an atom is found in the nucleus, rather than spread through the atom
Sketch this scientists model of the atom here: Name: Niels Bohr Date of discovery: 1913 What is the name of this scientist s model of the atom? The Bohr model Whose theory did this scientist build upon? Ernest Rutherford How did this scientist suggest electrons are arranged? In energy levels (shells) around the nucleus
QUESTIONS! Have a go at the left hand side of the worksheet using your notes if you need to Identify: For each of these models of the atom, identify its discoverer from the list below: Name: Democritus was a Greek philosopher that predicted the existence of the a_____. He ____________________ ____________________ ____________________ suggested atoms could not be spilt, that is they were i________. ____________________ ____________________ ____________________ In modern times Thomson, developed the p____ p______ model of the atom, where ______ electrons fit into a positive ball of charge. ______ In experiments performed in 1911 Rutherford developed a new n_______ model of ____________________ ____________________ the atoms where electrons orbit a nucleus. ____________________ ____________________ Bohr improved this model by discovering that e________ orbited in specific ____________________ ____________________ s________ orbits around the nucleus. ______ Rutherford Bohr ______ Democritus Electrons atoms plum pudding nuclear spherical indivisible Thomson Extension: Give a brief description of each atom in your own words.
Name: Democritus was a Greek philosopher that predicted the existence of the atoms. He suggested atoms could not be spilt, that is they were indivisible. In modern times Thomson, developed the plum pudding model of the atom, where electrons fit into a positive ball of charge. In experiments performed in 1911 Rutherford developed a new nuclear model of the atoms where electrons orbit a nucleus. Bohr improved this model by discovering that electrons orbited in specific spherical orbits around the nucleus. Identify: For each of these models of the atom, identify its discoverer. Democritus. Rutherford nuclear Atoms are indivisible model. building blocks of all Electrons orbit a positive matter (stuff). nucleus. Bohr model. Thomson's plum Electrons orbit in well pudding. defined spherical orbits. Electrons are in a positive ball of charge. Extension: Give a brief description of each atom in your own words.
If Thomson's model were true we would expect all alpha particles to pass through the gold. If Rutherford s model were true we would expect most alpha particles to pass through the gold, but some to be deflected.
Explain: (a) An experiment, designed to investigate the plum pudding model, involved firing alpha particles at a thin gold foil. If the plum pudding model was correct, then the alpha particles would go straight through the gold foil. The results of the experiment were unexpected. Why did this experiment lead to a new model of the atom, called the nuclear model, replacing the plum pudding model? The experiments performed by Rutherford did not support Thomson's plum pudding model. Therefore a new nuclear model was developed. (b) Using the Rutherford nuclear model of the atom, explain the three paths, A, B and C. A most of atom is empty space so particles pass straight through B nucleus is positive (so repels alpha particles) C nucleus is a concentrated mass/charge which repels particles straight back