Understanding Ions and Their Importance in Your Body
Neutral atoms have the same number of protons and electrons, with the charge of the nucleus always positive. The mass number is the sum of protons and neutrons. The number of neutrons can be calculated by subtracting the atomic number from the mass number. Ions are atoms with a positive or negative charge due to gaining or losing electrons. They play a crucial role in various bodily functions, such as nerve impulses and muscle contractions, as electrolytes like sodium, potassium, and calcium are essential for maintaining balance. Valence electrons determine an element's chemical behavior, with elements in the same column having similar properties.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
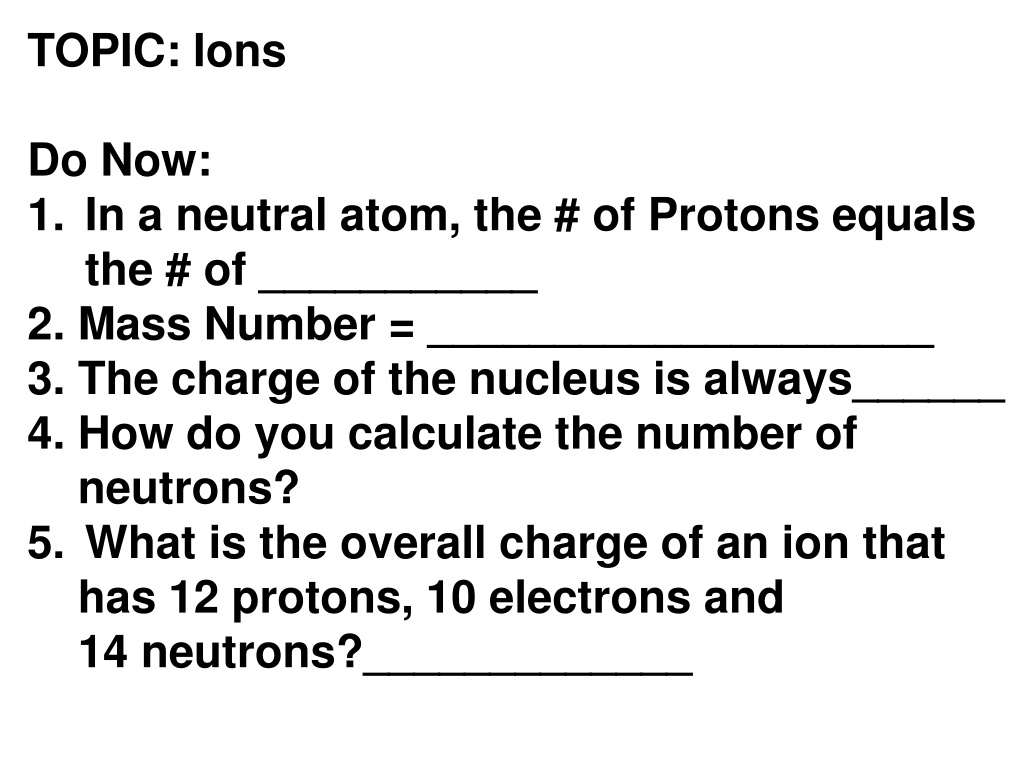
TOPIC: Ions Do Now: 1. In a neutral atom, the # of Protons equals the # of ___________ 2. Mass Number = ____________________ 3. The charge of the nucleus is always______ 4. How do you calculate the number of neutrons? 5. What is the overall charge of an ion that has 12 protons, 10 electrons and 14 neutrons?_____________
Atoms without charge are called ATOMS (they are neutral) Atoms with positive or negative charge are called IONS
WHEN REMOVING ELECTRONS THE RESULTING CHARGE IS POSITIVE 1+ neutral
WHEN ADDING ELECTRONS THE RESULTING CHARGE IS NEGATIVE 2 neutral
Metals lose electrons they become positive ions Nonmetals gain electrons they become negative ions
WHO CARES ABOUT IONS?!
ions make up the salt you put on your eggs in the morning - and many other compounds Ions are important for your body (we call them electrolytes Kidneys keep electrolyte concentration in the blood constant When you sweat you lose K+ and Na+ So you drink sports drinks Important to carry electrical impulses from the brain Nerve impulses Muscle contractions As for your body, the major electrolytes are as follows: sodium (Na1+) potassium (K1+) chloride (Cl1-) calcium (Ca2+) magnesium (Mg2+) bicarbonate (HCO3+) phosphate (PO42-) sulfate (SO42-)
1+ 2- 3+ 1- Why?!?!?!?!?!?! sodium (Na1+) potassium (K1+) chloride (Cl1-) calcium (Ca2+) magnesium (Mg2+) bicarbonate (HCO3+) sulfate (SO42-) It has to do with valance electrons!
Valence Electrons Chemical behavior determined by # valence electrons Elements with same # valence electrons will have similar chemical properties Elements in same column have similar chemical properties
Going Down Column 1: Period Element Configuration 1 H 1 2 Li 2-1 3 Na 2-8-1 4 K 2-8-8-1 5 Rb 2-8-18-8-1 6 Cs 2-8-18-18-8-1 7 Fr 2-8-18-32-18-8-1
Electron configuration 2-7 Inner electrons (kernel electrons) = 2 Valance electrons = 7 When predicting how Fluorine (F) will react, all we care about are the 7 valence electrons
Predicting Reactions Octet Rule: Everyone wants to be a noble gas. The noble gases are PERFECT! They have a full outer shell (8 valance electrons) 2-8 2-8-8 2-8-18-8 2-8-18-18-8 All elements must have a full outer shell in order to react, so they either take or share valence electrons. Atoms are lazy (like you!) and they don t want to do too much work. If they have 1,2,or 3 valence electrons, they will lose them rather than searching for 5,6,or 7 to fill their shell 1,2,3 valence electrons = lose 5,6,7 valence electrons = gain Remember 2 on the first shell is full
Lets Practice Element Configuration lost/gained Charge Config Electron # of e- New e Like which Noble Gas 1. Be 2-2 Lost 2 Be2+ 2 He 2. Al 2-8-3 Lost 3 Al3+ 2-8 Ne 3. S 2-8-6 Gain 2 S2- 2-8-8 Ar 4. Ba 2-8-18-18-8-2 Lost 2 Ba2+ 2-8-18-18-8 Xe 2-8-18-7 Gain 1 Br1- 2-8-18-8 Kr 5. Br 2-8-18-8-1 Lost 1 Rb1+ 2-8-18-8 Kr 6. Rb