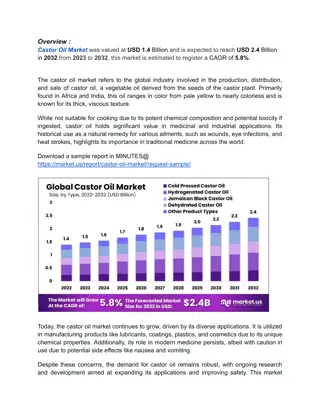
Validation Study of Rapeseed Oil Body Extraction Methods
This study explores the validation of two rapeseed oil body extraction methods. It delves into the common uses of rapeseed, the components of oil bodies, and their potential applications in the human food system. The materials and methods section details the extraction processes, including dry and wet grinding, centrifugation, and various treatments. The results highlight the analyses conducted on the extracted oil bodies. Overall, the objective is to find the method that yields the highest quantity of oil bodies with the highest purity.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Stephanie BLASER VALIDATION STUDY OF TWO RAPESEED OIL BODY EXTRACTION METHODS Marc ANTON, Eisabeth DAVID- BRIAND, Thibault LOISELEUX INRA 18/07/2014
INTRODUCTION Rapeseed Brassica napus Common uses Bulk oil - canola oil High protein animal feed Biodiesel
INTRODUCTION Oil bodies Energy storage structures in plant seeds 0.5- 2.5 m diameter Components Triaclyglycerol core Outer phospholipid monolayer Charged surface proteins - oleosins Structure gives physical and chemical stability
INTRODUCTION Potential applications in the human food system Deliver stable, preemulsifed oil into appropriate food systems Create a poorly-digested emulsion, through high pressure processing, for the increasing obese population Have an oil source extracted without solvents Objective Find the method that extracts the highest quantity of oil bodies with the highest purity
MATERIALS AND METHODS Extraction Method 3 Dry Grinding Wet Grinding Centrifugation 1 Centrifugation 2 Centrifugation 3 Collect Cream Carbonate Buffer (O.4 M sucrose) Carbonate Buffer (0.6 M sucrose) IKA Blender Polytron Blender Phosphate Buffer 40 g seeds 300 mg ground seeds 4 C 4 C 4 C 2 x 10 sec 2 x 15 sec 10000xg 10000xg 10000xg 8000 rpm 30 min 30 min 30 min
MATERIALS AND METHODS Extraction Method 5 Soak Seeds Dry Grinding Wet Grinding Centrifugation 1 Centrifugation 2 Centrifugation 3 Collect Cream 40 g seeds + 60 mL water Carbonate Buffer (0.4 M sucrose) Carbonate Buffer (0.6 M sucrose) IKA Blender Polytron Blender Phosphate Buffer Refrigerate overnight 2 x 10 sec 503 mg ground seeds 4 C 4 C 4 C 2 x 15 sec 10000xg 10000xg 10000xg 8000 rpm 30 min 30 min 30 min
MATERIALS AND METHODS Extraction 3 and 5 treatments Regular - fresh cream in phosphate buffer (100 mg/ml) Freeze-dried cream in phosphate buffer Freeze-dried cream in water Analyses Dry Matter Size Microscope Granulometer Nanosizer Protein quantification BSA Lipid quantification Isopropanol/hexane extraction
CREAM WEIGHTS Average Cream Average Cream Weights (mg) Weights (mg) Method Method Standard Deviation Standard Deviation Sample Sample Size Size 3 28 100,84 15,24 5 14 110,03 15,88
LIGHT MICROSCOPE Method 3 Method 5 63x 63x
CONFOCAL MICROSCOPE Method 3
GRANULOMETER Average Particle Size Average Particle Size 7 6 5 Method 3 Regular 4 Volume (%) Volume (%) Method 3 Freeze Dried with Buffer Method 3 Freeze Dried with Water 3 Method 5 Regular Method 5 Freeze Dried with Buffer 2 Method 5 Freeze Dried with Water 1 0 0.1 1 10 100 1000 Diam tre des gouttes ( m) Diam tre des gouttes ( m)
NANOSIZER METHOD 3 Regular Freeze-dried
NANOSIZER METHOD 5 Regular Freeze-dried
LIPID QUANTIFICATION Sample Sample Size Size Average Lipid Average Lipid (mg) (mg) Standard Standard Deviation Deviation Method Method Treatment Treatment Regular 7 7,19 1,10 Freeze-Dried with Buffer 2 5,70 - 3 Freeze-Dried with Water 2 7,00 - Overall 11 6,89 2,22 Regular 2 6,35 - Freeze-Dried with Buffer 2 6,84 - 5 Freeze-Dried with Water 4 10,28 2,06 Overall 8 8,43 2,40
PROTEIN QUANTIFICATION Average Average Concentration Concentration (mg/ml) (mg/ml) Sample Sample Size Size Standard Standard Deviation Deviation Method Method Treatment Treatment Regular 3 2,46 ,51 Freeze-Dried with Buffer 1 1,32 - 3 Freeze-Dried with Water 1 1,36 - Overall 5 2,01 ,71 Regular 1 5,01 - Freeze-Dried with Buffer 1 1,84 - 5 Freeze-Dried with Water 2 3,62 - Overall 4 3,52 1,39
DRY MATTER Sample Sample Size Size Average Percent Average Percent Dry Matter (%) Dry Matter (%) Standard Standard Deviation Deviation Method Method Treatment Treatment Dry Heat 4 52,18 1,80 Freeze-Drying with Buffer 2 55,59 - 3 Freeze-Drying with Water Overall Overall Freeze-Drying with Buffer Freeze-Drying with Water Overall Overall 2 8 8 1 3 4 4 55,26 54,35 54,35 58,63 44,84 48,29 48,29 - 1,88 1,88 - 3,17 7,36 7,36 5
DISCUSSION Cream Collection Weights No major difference between Method 3 and 5 Size Method 3 vs 5 Both displayed similar presence of floculation and particle sizes- granulometer, nanosize, and light microscope Regular vs. Freeze-dried Light microscope No major visual differences Granulometer More floculation present in samples freeze-dried with buffer Nanosizer Freeze-dried samples showed a wider range of particle sizes Differences in freeze-dried samples potentially due to destruction of oil bodies during harsh treatment
DISCUSSION Lipid Quantification Slightly higher collection from Method 3 Protein Quantification Slighly lower concentration from Method 3 Dry Matter Slightly higher Percent Dry Matter from Method 3
DISCUSSION Challenges Cream collection Cream can stick to cap of centrifuge tube Cream in Method 3 is, in general, not as firm and durable as Method 5 Size measurement instrumentation Granulometer and nanometer have size detection limits that are on both sides of the oil bodies upper and lower diameter range New equipment arriving next month Freeze-drying Low volume of final product Time consuming Slightly lower protein content With buffer Powdery final product - easier to collect Contained phosphate buffer salts Room for errors in calculations With water Waxy final product - more difficult to collect
SUSTAINABILITY Future options Using sustainabiliy grown seeds 2010 Unilever Sustainable Agriculture Code Put into effect in Germany under Cargill Using the valueable protein for a human food source and not just for animal meal Hurdles: glucosinolates, phenolics, phytates, and high amount of fiber Benefits: balanced amino acid profile, functional properties (emulsifying, foaming, and gelling), and new alternative to feed increasing population Creating industrial scale extraction methods without the use of dangerous solvents
CONCLUSION Method 3 More consistent Less time consuming Less protein contamination Method 5 Easier handling of cream Long soak step More protein contamination Freeze-drying Time consuming Few added benefits Next step Compare against Thibault s data Increase collection volume to larger bench scale
THANK YOU Stephanie Jung, PhD Marc Anton, PhD Elisabeth David-Briand Thibault Loiseleux