Valence Electrons and Lewis Dot Diagrams
Explore the concept of valence electrons and Lewis dot diagrams in chemistry. Learn how to identify the number of protons, neutrons, and electrons in an element using Bohr model drawings. Discover the significance of valence electrons in bonding and how to determine the number of valence electrons for different elements.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Bohr Model Drawings Identify the number of Protons, Neutrons, and Electrons in an element. (PEN) Write the number of Protons and Neutrons in the center of the nucleus. Draw the number of electrons in each of the orbitals. (PEN)
Bohr Model Drawings Carbon P: E: N: P: N:
Valence Electrons and Lewis Dot Diagrams
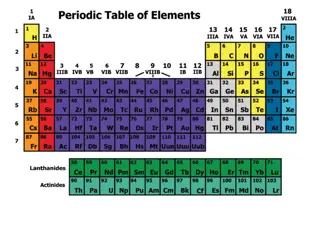
Groups Groups or Families: or Families: Vertical Columns of the Periodic Table Period Period or Series: or Series: Horizontal Rows of the Periodic table
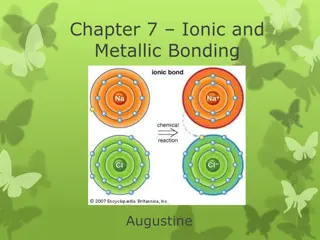
Valence electrons Valence electrons farthest away from the nucleus. Every group of elements will have a different number of valence electrons electrons are the
Why are valence electrons important? Valence electrons are key to whether or not an atom bonds with another atom The number of valence electrons is the face an atom shows to other atoms One way to show valence electrons is with electron dot diagrams or Lewis dot diagrams
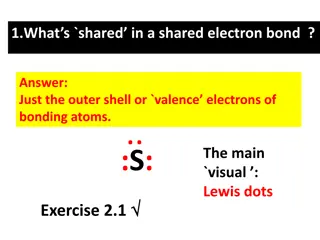
Lewis dot diagrams Lewis dot diagrams include the symbol for the element surrounded by dots. Each dot stands for one valence electron Hydrogen Hydrogen H Nucleus
How to find the # of valence electrons Find out which group (column) your element is in. This will tell you the number of valence electrons your element has. You will only draw the valence electrons.
Groups Elements in group 1 will have only 1 valence electron Group 1 = 1 electron
Groups Elements in group 2 will have only 2 valence electron Group 2 = 2 electrons
Groups Elements in group 3 will have only 3 valence electron Group 3 = 3 electrons
Groups Elements in group 4 will have only 4 valence electron Group 4 = 4 electrons
Groups Elements in group 5 will have only 5 valence electron Group 5 = 5 electrons
Groups Elements in group 6 will have only 6 valence electron Group 6= 6 electrons
Groups Elements in group 7 will have only 7 valence electron Group 7 = 7 electrons
Groups Elements in group 8 will have only 8 valence electron Group 8 = 8 electrons Except for He, it has 2 electrons
Groups - Review Group 8 = 8 electrons Group 1 = 1 electron Except for He, it has 2 electrons Group 2 = 2 electrons 3, 4, 5, 6, 7
How to: Lewis Structures 1) Find your element on the periodic table. 2) Determine the number of valence electrons. 3) This is how many electrons you will draw. 4) Draw them starting at the right, and in a counterclockwise manner.
Lewis Structures 1) Write the element symbol. C
Lewis Structures 1) Write the element symbol. 2) Carbon is in the 4th group, so it has 4 valence electrons. C
Lewis Structures 1) Write the element symbol. 2) Carbon is in the 4th group, so it has 4 valence electrons. 3) Starting at the right, draw 4 electrons, or dots, counter- clockwise around the element symbol. C
Electron Cloud Electron Cloud