Lewis Dot Structures and Polarity Review
Learn about Lewis dot structures, how to draw them, determine polarity, and practice drawing various compounds. Understand the concepts of valence electrons, central atoms, bonding, and lone pairs in molecules. Discover the distinction between polar and nonpolar structures based on symmetry.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Lewis Dot Structures & Polarity Review
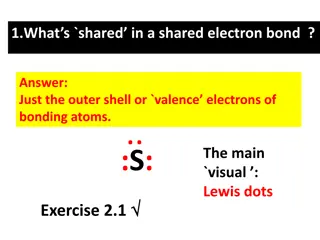
Dot Diagrams vs. Structures Dot Diagrams vs. Structures Lewis Dot Diagrams show the number of valence of electrons electrons (in the form of dots) for a single element Lewis Structures are diagrams that show the bonding between atoms of a molecule and the lone pairs that may exist in the molecule
Drawing Lewis Structures Review 1. Count up the valence electrons Anions: add e (CO32-: add 2 e ) Cation: subtract e (NH4+: minus 1 e ) 2. Determine the central atom Usually the first element is in the center (often C, never H) 3. Bond Terminal Atoms (update electron count) 4. Place remaining electrons around atoms in lone pairs (starting outside first) Place electrons in pairs (lone pairs) Too few? Form multiple bonds between atoms: double bond (4 e-) and triple bond (6 e-) 5. Check your structure! Exceptions: Remember that H only needs 2e !
Lewis Structure Useful Hints C group: Forms a combo of 4 bonds and no LP O (0) (0) C (0) (Lone Pairs) O N H H H N group: Forms a combo of 3 bonds and 1 LP O group: Forms a combo of 2 bonds and 2 LP O F F F group (halogens): Forms 1 bond and 3 LP Note that these are NOT always true!
Lewis Structures Practice Lewis Structures Practice Practice drawing the following compounds in your notebook. CO2 NH3 HCl CH4
Lewis Structures Practice Lewis Structures Practice - - ANSWERS NH3 ANSWERS CH4 CO2 HCl
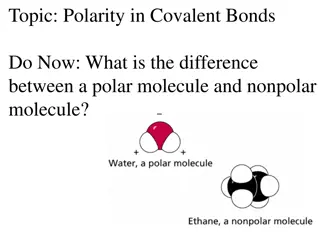
How do you determine if a structure is polar or nonpolar? A structure will be nonpolar if is symmetrical around the central atom. A structure will be polar if it is asymmetrical around the central atom.
Identify the following structures as polar or nonpolar. NONPOLAR POLAR POLAR NONPOLAR POLAR


![[PDF⚡READ❤ONLINE] Planet Mercury: From Pale Pink Dot to Dynamic World (Springer](/thumb/21549/pdf-read-online-planet-mercury-from-pale-pink-dot-to-dynamic-world-springer.jpg)