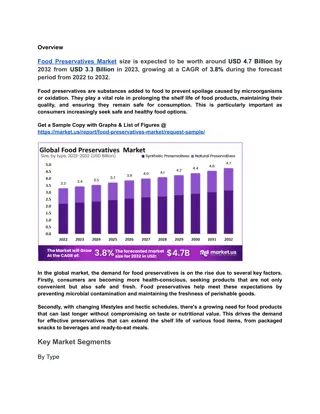
Understanding Vaccine Components and Preservatives
Vaccine components such as antigens and adjuvants play a crucial role in helping the immune system fight off infections effectively. Preservatives like thimerosal and phenol are used to prevent microbial growth in vaccines and ensure their safety. While the use of preservatives has declined with advancements in manufacturing, they still play a vital role in vaccine production and storage.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Provide immunity Antigens Adjuvants Keep vaccines safe and long lasting Preservatives Stabilizers Used during the production of vaccines Cell culture materials Inactivating ingredients Antibiotics
VACCINE COMPONENTS Antigens are very small amounts of weak or dead pathogens that can cause diseases. They help the immune system learn how to fight off infections faster and more effectively. The influenza virus is an example of an antigen. Adjuvants, which are in some vaccines, are substances that help the immune system respond more strongly to a vaccine. This increases the immunity against the disease. Aluminum is an example of an adjuvant (Aluminum is the world s most abundant metal, found in plants, soil, water and air. The quantities of aluminum present in vaccines are low and highly regulated)
VACCINE COMPONENTS Preservatives may be defined as compounds that kill or prevent the growth of microorganisms, particularly bacteria and fungi. They are used in vaccines to prevent microbial growth in the event that the vaccine is accidentally contaminated, as might occur with repeated puncture of multi-dose vials with a needle. In some cases, preservatives are added during the manufacturing process to prevent microbial growth. However, improvements in manufacturing technology have markedly decreased the need to add preservatives during the manufacturing process.
Preservatives Thimerosal : is a mercury-containing organic compound (an organomercurial). Since the 1930s, it has been widely used as a preservative in a number of biological and drug products, including many vaccines, to help prevent potentially life threatening contamination with harmful microbes. The documented antimicrobial properties of thimerosal contribute to the safe use of vaccines in multi-dose vials, and the ability to package certain vaccines, such as those for seasonal and pandemic influenza, in multi-dose vials helps facilitate immunization .
Preservatives A vaccine containing 0.01% thimerosal as a preservative. For comparison, this is roughly the same amount of elemental mercury contained in a 3 ounce can of tuna fish. Thimerosal in concentrations of 0.001% (1 part in 100,000) to 0.01% (1 part in 10,000) has been shown to be effective in clearing a broad spectrum of pathogens While the use of mercury-containing preservatives has declined in recent years due to the development of new products formulated into single-dose presentations that do not require preservatives, thimerosal has been used in some immune globulin preparations, anti-venins, skin test antigens, and ophthalmic and nasal products, in addition to some vaccines.
Preservatives phenol : currently used as a preservative in three FDA-approved available vaccines, Pneumovax 23 (for prevention of pneumococcal disease caused by the 23 serotypes contained in the vaccine) and TyphimVi (for prevention of typhoid fever) and ACAM2000 (for prevention of smallpox); each of these vaccines contains 0.25% phenol. These vaccines are not recommended for routine use by the Centers for Disease Control and Prevention s (CDC) and Advisory Committee on Immunization Practices (ACIP). Phenol is used in a variety of consumer products including mouthwashes, throat lozenges, and throat sprays.
Preservatives Benzethoniumchloride : is a chemical that has antimicrobial properties. It is used in over-the-counter hand and body washes. This preservative is currently used in only one FDA approved vaccine, BioThrax, for the prevention of disease caused by Bacillus anthracis.
Stabilizers like sugar or gelatin, help the active ingredients in vaccines continue to work while the vaccine is made, stored. and moved Sometimes, trace amounts of some ingredients that are needed to produce the vaccine remain in the final product. Examples of these non-harmful ingredients include: Cell culture (growth) material, like eggs, to help grow the vaccine antigens Inactivating (germ-killing) ingredients, like formaldehyde, to weaken or kill viruses, bacteria or toxins in the vaccine Antibiotics, like neomycin, to help keep outside germs and bacteria from growing in the vaccine
Types of vaccines A heterologous vaccine (also known as a "Jennerian" vaccine) is a type of live vaccine where one pathogen is introduced in order to provide protection against a different one. The vaccines are pathogens of other animals that either do not cause disease or cause mild disease in the organism being treated. Examples :Jenner's administration of cowpox (vaccinia) to protect against smallpox (variola); BCG vaccine made from Mycobacterium bovis to protect against human tuberculosis.
Homologous vaccine are those which are produced from one and used against the same. The vaccine can be classified in to: A- Routine vaccines: are well tolerated provide adequate protection against dangerous and widespread infection e.g diphtheria, polio, measles, mumps. Most people think of these as childhood vaccines that you get before starting school, but some vaccines are routinely recommended for adults, and some are recommended every year (a flu vaccine) or every 10 years (a tetanus booster). B- Indicated vaccines: are performed only under certain conditions, tourist vaccines belong to this group.
Vaccine Considerations Safety Immunogenicity Type of protection Time quickness longevity Route of administration Magnitude Antigenic variation Target population Storage Cost Most vaccines in current use work by inducing humoral immunity.
Vaccine Benefits Protection or Immunity Reduced exposure Disease prevention Individual Community Herd immunity Cost
Factors affecting immunization 1- Genotype of Recipient a major factor that determines immune responsiveness. Some substances are immunogenic in one species but not in another. Similarly, some substances are immunogenic in one individual but not in others. MHC gene products which function in processing and presenting of antigen influence response towards antigen. Genes that codes B-cell and T-cell receptor also influences immunogenicity. Also, the genes coding proteins for various regulatory mechanisms influences it. 2-Dosage and Route of administration: An insufficient dosage of immunogen will not elicit an immune response (Either it fails to elicit immune response or lead to a state of tolerance). Conversely, an excessively high dose will also lead to unresponsiveness or tolerance.
Factors affecting immunization single dosage is not sufficient to develop immune response ,however booster dose over a period of time enhances immunogenicity. Administration route strongly influences the immune response (e.g. orally; injected into the skin; inhaled intranasally), the subcutaneous route is better than the intravenous or intragastric routes. 3-Adjuvants:Adjuvants (taken from the Latin, adjuvare, meaning to help ) are designed to improve poorly immunogenic vaccines. One way to increase the immunogenicity of a vaccine is introduce additional material specifically to provoke a stronger immune response.. These are substances that when mixed and injected with antigen enhances the immunogenicity of antigen. The group of adjuvants in most common use, aluminum salts.
Adjuvants Aluminium potassium sulphate (alum) is an adjuvant that enhances immunogenicity by increasing persistence of antigen (Releasing antigen slowly from injection site) and enhancing phagocytosis of antigen.TLR interaction and cytokine induction. water-in-oil or oil-in-water emulsions (e.g. Freund s adjuvant), as well as natural and synthetic toxins derived from bacteria (e.g. cholera toxin, CT and lymphotoxin, LT). Slow release of antigen. Freund's adjuvant is a solution of antigen emulsified in mineral oil and used as an immunopotentiator (booster). A-The complete form, Freund's Complete Adjuvant (FCA or CFA) is composed of inactivated and dried mycobacteria (usually M. tuberculosis), whereas.
Adjuvants B- the incomplete form (FIA or IFA) lacks the mycobacterial components (hence just the water in oil emulsion). The use of adjuvants, however, is often hampered by undesirable side effects such as fever and inflammation. Adjuvants enhance immunogenicity in various ways: 1-Prolonged persistence of antigen, and increase the immunogenicity of weak antigens. 2-Co-stimulatory signals are prolonged, and enhance the speed and duration of the immune response. 3-Local inflammatory response is increased, and stimulates and modulates humoral responses, stimulate cell-mediated immunity, and improve the induction of mucosal immunity. 4-Non-specific proliferation of lymphocytes is increased.
Other issues associated with immunization A) Antigenic variation. Influenza virus, for example, can rapidly alter its antigenic structure by mutation, so that it is no longer recognized by antibodies made against the original virus. Influenza can, therefore, give rise to repeated infection with variants of the same organism. B) Antigenic competition. Two antigens given at the same site can sometimes each interfere with the immune response to the other. In general, therefore, different immunizations are given at different times and/or at different sites. But remember that DPT is a notable exception to this rule, and other routine multiple vaccination protocols exist (such as MMR). C) Maternal immunoglobulin. The presence of specific antibodies at the time of vaccination may interfere with its success. The measles vaccine, for example, should not be given before 15 months of age, since the presence of maternal IgG antibodies may prevent active immunization.
Plasma therapy Plasma is the largest part of blood. It makes up more than half (about 55%) of its overall content. When separated from the rest of the blood, plasma is a light yellow liquid. The main role of plasma is to take nutrients, hormones, and proteins to the parts of the body that need it. Cells also put their waste products into the plasma. The plasma then helps remove this waste from the body. Blood plasma also carries all parts of the blood through your circulatory system. Along with water, salt, and enzymes, plasma also contains important components. These include antibodies, clotting factors, and the proteins albumin and fibrinogen. proteins and antibodies in plasma are also used in therapies for rare chronic conditions. These include autoimmune disorders and haemophilia. Several countries are seriously looking at plasma therapy as a potential treatment for Covid-19, the disease caused by the novel coronavirus. Plasma therapy uses blood donated by recovered patients to introduce antibodies in those under