Understanding the Periodic Table: Elements, Trends, and Atomic Radius
The periodic table organizes elements by properties, with Mendeleev's contributions and predictions. Elements are grouped into periods and groups, displaying trends in ionization energy, electronegativity, and atomic size. Learn about the atomic radius, how it varies across periods and groups, influencing the size of atoms and ions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Chapter 6 The Periodic Table
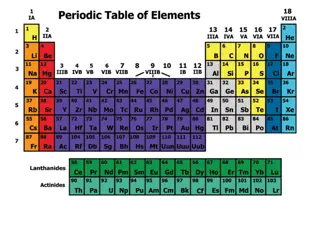
Periodic table Elements are arranged based on similarities in their properties Dmitri Mendeleev is credited with our current periodic table Even predicted the existence of elements that were not yet discovered(i.e. Gallium) His periodic table was slightly revised a few years later so that the elements were in order of increasing atomic number Can be broken down into groups (columns) and periods (rows)
Periods Range from 1-7 Indicates the highest occupied energy level in an atom
Groups 2 numbering systems: 1-18 and A/B elements We will be using the A/B designation A-elements are called the representative elements (besides noble gases) B-elements are the transition and inner transition metals A-group names: I A- alkali metals II A- alkaline earth metals VII A- halogens VIII A- noble gases
Zig-zag line Locate the zig-zag line starting at III A Metals are to the left of the line Nonmetals are to the right of the line Metalloids are those elements that border the line (except Al !!)
Color of the symbol Black (includes white outlined in black) solid at room temp. Blue liquid at room temp Red gas at room temp
Periodic Trends Chapter 6
Standards Students know how to use the periodic table to identify trends in ionization energy, electronegativity, and the relative sizes of ions and atoms.
Atomic Radius Definition: Half of the distance between nuclei in covalently bonded diatomic molecule Radius decreases across a period Increased effective nuclear charge Radius increases down a group Each row on the periodic table adds a shell or energy level to the atom
Table of Atomic Radii
Period Trend: Atomic Radius
Ionization Energy Definition: the energy required to remove an electron from an atom Tends to increase across a period As radius decreases across a period, the electron you are removing is closer to the nucleus and harder to remove Tends to decrease down a group Outer electrons are farther from the nucleus and easier to remove
Periodic Trend: Ionization Energy
Electronegativity Definition: A measure of the ability of an atom in a chemical compound to attract electrons oTends to increase across a period o As radius decreases, electrons get closer to the bonding atom s nucleus o Tends to decrease down a group oAs radius increases, electrons are farther from the bonding atom s nucleus
Periodic Trend: Electronegativity
Summary of Periodic Trends
Ionic Radii Positively charged ions formed when an atom of a metal loses one or more electrons Smaller than the corresponding atom Cations Negatively charged ions formed when nonmetallic atoms gain one or more electrons Larger than the corresponding atom Anions
Table of Ion Sizes
Practice Identify each of the following: Which has a larger atomic radius? F , O Na , Li Which has a higher ionization energy? Li , B Mg , Sr Which has a higher electronegativity value? Na , K C , N Which particle has to largest radium in each atom/ion pair? Na , Na+ S , S-2