Understanding S-Block Elements in the Periodic Table
The s-block elements in the Periodic Table consist of 14 elements with unique properties and characteristics. Lithium, sodium, and potassium are notable members of Group 1, characterized by their reactivity and ability to form alkaline solutions when in contact with water. These soft metals exhibit low density, can be cut with a knife, have low melting points, and are good conductors of heat and electricity. The s-block neighborhood in the Periodic Table houses elements unified by their s-orbital valence electrons, making them some of the most reactive elements. Explore more about the distinct properties and behavior of s-block elements in this informative guide.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
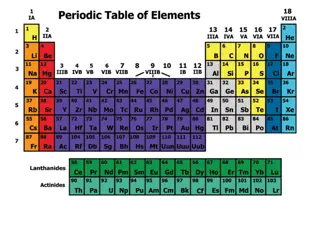
S S- -Block Elements Block Elements The s-block elements include hydrogen (H), helium (He), lithium (Li), beryllium (Be), sodium (Na), magnesium (Mg), potassium (K), calcium (Ca), rubidium (Rb), strontium (Sr), cesium (Cs), barium (Ba), francium (Fr) and radium (Ra). The periodic table shows exactly where these elements are within the s-block.
Lithium, sodium and potassium all belong to Group 1. Lithium, sodium and potassium all belong to Group 1. Properties: Properties: Soft metals that can be cut with a knife. Soft metals that can be cut with a knife. Low density Low density - - can float on water. can float on water. Low melting points in comparison with other metals. Low melting points in comparison with other metals. They react violently (in some cases) with water to form alkaline solutions They react violently (in some cases) with water to form alkaline solutions - - hence the name, alkali metals. hence the name, alkali metals. Potassium is more reactive than lithium, since although they both need to lose one electron to have full outer shells, potassium's outer electron is furthest from the positive attractions of the nucleus. Therefore, it is easier for potassium to lose its outer electron than it is for lithium. Melting point and boiling point decreases down the group. If the periodic table were a city, the s-block would be a small neighborhood filled with extremely similar houses and properties. Within the periodic table, the s-block is located to the far left and includes all of the elements in the first two columns (columns 1 and 2) plus helium, which is located in the top right corner in column 8A (column 18 on some versions of the periodic table). In the periodic table below, the s- block is colored pink.
The s-block elements are the 14 elements contained within these columns. All of the s-block elements are unified by the fact that their valence electrons (outermost electrons) are in an s orbital. The s orbital is spherical and can be occupied by a maximum of two electrons. Elements in column 1 have one electron in the s orbital, and elements in column 2 (plus helium) have two electrons in the s orbital. The s-block elements include hydrogen (H), helium (He), lithium (Li), beryllium (Be), sodium (Na), magnesium (Mg), potassium (K), calcium (Ca), rubidium (Rb), strontium (Sr), cesium (Cs), barium (Ba), francium (Fr) and radium (Ra). The periodic table shows exactly where these elements are within the s-block.
Properties of S-Block Elements If the elements were houses in our hypothetical s-block neighborhood, they would be very uniform, each one only slightly different than the other. This is markedly different compared to the other neighborhoods on the periodic table, which have a wider variety of houses in many shapes, sizes and colors. All of the s-block elements are metals. In general, they are shiny, silvery, good conductors of heat and electricity and lose their valence electrons easily. In fact, they lose their trademark s orbital valence electrons so easily that the s-block elements are considered to be some of the most reactive elements on the periodic table. The elements in column 1, known collectively as the alkali metals (except hydrogen), always lose their one valence electron to make a +1 ion. These metals are characterized by being silvery, very soft, not very dense and having low melting points. These metals react extremely vigorously with water and even oxygen to produce energy and flammable hydrogen gas. They are kept in mineral oil to reduce the chance of an unwanted reaction or worse, an unwanted explosion. The elements in column 2, known as the alkaline earth metals (except helium), always lose their two valence electrons to make a +2 ion. Like the alkali metals, the alkaline earth metals are silvery, shiny and relatively soft. Some of the elements in this column also react vigorously with water and must be stored carefully. S-block elements are famous for being ingredients in fireworks. The ionic forms of potassium, strontium and barium make appearances in firework displays as the brilliant purples, reds and greens.
Properties of s- elements All of the s- elements are metals(except Hydrogen&Helium). In general, they are shiny, silvery, good conductors of heat and electricity. They lose their valence electrons easily. In fact, they lose their trademark s orbital valence electrons so easily that the s- elements are some of the most reactive elements on the periodic table. The elements in group 1, known collectively as the alkali metals (except hydrogen), always lose their one valence electron to make a +1 ion. These metals are characterized by being silvery, very soft, not very dense and having low melting points. These metals react extremely vigorously with water and even oxygen to produce energy and flammable hydrogen gas. They are kept in mineral oil to reduce the chance of an unwanted reaction or worse, an explosion. The elements in group 2, known as the alkaline earth metals (except helium), always lose their two valence electrons to make a +2 ion. Like the alkali metals, the alkaline earth metals are silvery, shiny and relatively soft. Some of the elements in this column also react vigorously with water and must be stored carefully. S- elements are famous for being ingredients in fireworks. The ionic forms of potassium, strontium and barium make appearances in firework displays as the brilliant purples, reds and greens. Francium is considered to be the most rare naturally occurring element on earth. It is estimated that there is only ever one natural atom of Francium present on earth at a time. Francium has a very unstable nucleus and undergoes nuclear decay rapidly.
Chemical properties of alkali metals 1.Alkali metals react with dry hydrogen to form hydrides. a.These hydrides are ionic in nature b.These hydrides of alkali metals react with water to form corresponding hydroxides and hydrogen gas. LiH+ H2O->LiOH+H2 c.These hydrides are strong reducing agents and their reducing nature increases down the group. d.Alkali metals also form complex hydrides such as LiAlH4which is a good reducing agent.Alkali metal hydrides do not exist in water and this reaction with any other agent is carried out in protic solvent. e.Fused alkali metal hydrides on electrolysis produce H2gas at anode. 2.Formation of oxides and hydroxides. a.These are most reactive metals and have strong affinity towards O2 ,they form oxides on surface.They are kept under kerosene or paraffin oil to protect them from air. b.When burnt in air (O2) ,li forms Li20 , Na forms Na2O2and other alkali metals form superoxides. 3. They are purely metallic , as they lose the electrons from the outermost shell readily , they are highly reactive metals and they have low ionization energy . 4. Beryllium is amphoteric in nature .
Diagonal relationship The first element in group one, Lithium, and the first in group two, Beryllium, behave differently to other members of their groups. Their behaviour is like the second element of the next group. So lithium is similar to magnesium, and beryllium is similar to aluminumIn the periodic table this is known as a 'diagonal relationship'. The diagonal relationship is because of similarities in ionic sizes and charge/radius ratio of the element. The similarity between lithium and magnesium is because of their similar sizes Radii, Li=152pm Mg=160pm Group 1 2 13 14 Period 2 Li Be B C Period 3 Na Mg Al Si
Lithium Lithium has many different behaviours to other elements in group one. This difference caused by: the small size of the lithium atom and its ion. the higher polarization power of li + (i.e. charge size ratio). This means increased covalent character of its compounds which is responsible for their solubility in organic solvents high ionisationenthalpy and high electronegative character of lithium as compared to other alkali metals non availability of d-orbitals in its valence shell strong intermetallic bonding Some of the ways in which lithium behaves differently from other members of are:[source?] Lithium is harder than sodium and potassium which are so soft that they can be cut by a knife. The melting and boiling points of lithium are higher. Lithium forms monoxidewith oxygen, other alkali form peroxideand superoxide. Lithium combines with nitrogen to form nitrides, while other alkali metals do not. Lithium Chloride is deliquescentand crystallizes as a hydrate LiCl.2H2O. Other alkali metal chlorides do not form hydrates.
Sodium - Na Sodium (Na),chemical element of the alkali metal group (Group 1 [Ia]) of the periodic table. Sodium is a very soft silvery-white metal. Sodium is the most common alkali metal and the sixth most abundant element on Earth,comprising2.8 percent of Earth s crust. It occurs abundantly in nature in compounds, especially common salt sodium chloride (NaCl) which forms the mineral haliteand constitutes about 80 percent of the dissolved constituents of seawater. 22.98977 g.mol-1 Atomic mass Electronegativity according to Pauling 0.9 0.97 g.cm-3at 20 C Density Melting point 97.5 C Boiling point 883 C Vanderwaals radius 0.196 nm Ionic radius 0.095 (+1) nm Isotopes 3 [Ne] 3s1 Electronic shell 495.7 kJ.mol-1 Energy of first ionisation Standard potential - 2.71 V Discovered by Sir Humphrey Davy in 1807
Chemical element, symbol: Na, atomic number: 11 and atomic weight 22,9898. Its a soft metal, reactive and with a low melting point, with a relative density of 0,97 at 20 C (68 F). From the commercial point of view, sodium is the most important of all the alkaline metals. Sodium reacts quickly with water, and also with snow and ice, to produce sodium hydroxide and hydrogen. When it s exposed to air, metallic sodium recently cut looses its silvery appearance and acquires an opaque grey colour due to the formation of a sodium oxide coating. Sodium doesn t react with nitrogen, not even at very high temperatures, but it can react with ammonia to form sodium amide. Sodium and hydrogen react above 200 C (390 F) to form sodium hydride. Sodium hardly reacts with carbon, but it does react with halogens. It also reacts with various metallic halides to form the metal and sodium chloride. Sodium doesn t react with paraffinic hydrocarbons, but it forms addition compounds with naphthalene and other aromatic polycyclic compounds and with aryl alkenes. The reaction of sodium with alcohols is similar to the reaction of sodium with water, but slower. There are two general reactions with organic halides. One of them requires the condensation of two organic compounds, which form halogens when those are eliminated. The second type of reaction includes the replacement of halogen by sodium, to obtain a sodium organic compound. Applications Sodium in its metallic form is very important in making esters and in the manufacture of organic compounds. Sodium is also a component of sodium chloride (NaCl) a very important compount found everywhere in the living environment. Other uses are: to improve the structure of certain alloys; in soap, in combination with fatty acids, in sodium vapor lamps, to descal metals, to purify molten metals. Solid sodium carbonate is needed to make glass.
alkali metal any of the six chemical elements that make up Group 1 (Ia) of the periodic table namely, lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). The alkali metals are so called because reaction with water forms alkalies (i.e., strong bases capable of neutralizing acids). Sodium and potassium are the sixth and seventh most abundant of the elements,... Lighter than water, sodium can be cut with a knife at room temperature but is brittle at low temperatures. It conducts heat and electricity easily and exhibits the photoelectric effect (emission of electrons when exposed to light) to a marked degree. Sodium is by far the most commercially important alkali metal. Most processes for the production of sodium involve the electrolysis of molten sodium chloride. Inexpensive and available in tank-car quantities, the element is used to produce gasoline additives,polymers such as nylon and synthetic rubber, pharmaceuticals, and a number of metals such as tantalum,titanium, and silicon. It is also widely used as a heat exchanger and in sodium-vapour lamps. The yellow colour of the sodium-vapour lamp and the sodium flame (the basis of an analytical test for sodium) is identified with two prominent lines in the yellow portion of the light spectrum.
Health effects of sodium Sodium is a compound of many foodstuffs, for instance of common salt. It is necessary for humans to maintain the balance of the physical fluids system. Sodium is also required for nerve and muscle functioning. Too much sodium can damage our kidneys and increases the chances of high blood pressure. The amount of sodium a person consumes each day varies from individual to individual and from culture to culture; some people get as little as 2 g/day, some as much as 20 grams. Sodium is essential, but controversely surrounds the amount required. Contact of sodium with water, including perspiration causes the formation of sodium hydroxide fumes, which are highly irritating to skin, eyes, nose and throat. This may cause sneezing and coughing. Very severe exposures may result in difficult breathing, coughing and chemical bronchitis. Contact to the skin may cause itching, tingling, thermal and caustic burns and permanent damage. Contact with eyes may result in permanent damage and loss of sight. Environmental effects of sodium Sodium's powdered form is highly explosive in water and a poison combined and uncombined with many other elements. Ecotoxicity: Median tolerance limit (TLM) for the mosquito fish, 125 ppm/96hr (fresh water); Median tolerance limit (TLM) for the bluegill, 88 mg/48hr (tap water). Environmental fate: this chemical is not mobile in solid form, although it absorbs moisture very easily. Once liquid, sodium hydroxide leaches rapidly into the soil,
Potassium is one of the alkali metals. The alkali metals are the elements that make up Group 1 (IA) of the periodic table. The periodic table is a chart that shows how chemical elements are related to one another. The alkali metals also include lithium, sodium, rubidium, cesium,and francium.They are among the most active metals. Potassium is so active that it never occurs free in nature. It always occurs in compounds, combined with other elements. It was first prepared in pure form in 1807 by English chemist Sir Humphry Davy (1778-1829). Davy used a new method of isolating elements that he had invented, electrolysis. In electrolysis, an electric current is passed through a molten (melted) compound. The electrical current breaks the compound into its elements. (See sidebar on Davy in the calcium entry in Volume 1.) There are very few uses for potassium as a pure element. However, compounds of potassium have many important applications, the most important of which is as a fertilizer.
SYMBOL K ATOMIC NUMBER 19 19 ATOMIC MASS 39.0983 FAMILY Group 1 (IA) Physical properties Potassium is a soft, silvery-white metal with a melting point of 63 C (145 F) and a boiling point of 770 C (1,420 F). Its density is 0.862 grams per cubic centimeter, less than that of water (1.00 grams per cubic centimeter). That means that potassium metal can float on water. Chemically, though, that's not a good idea (see "Chemical properties" below). The melting point of potassium is very low for a metal. It will melt over the flame of a candle flame Chemical properties Like the other alkali metals, potassium is very active. It reacts with water violently and gives off hydrogen gas: So much heat is produced in this reaction that the hydrogen gas actually catches fire and may explode. Floating potassium metal on the surface of water is not a good idea! In that instance, the potassium would skip along the surface of the water. The skipping is caused by hydrogen gas produced in the reaction pushing the metal around. The potassium would soon catch fire, burn, and, perhaps, explode. Potassium reacts readily with all acids and with all non-metals, such as sulfur, chlorine, fluorine, phosphorus,and nitrogen.
Potassium occurs widely in many different minerals. Some of the most important of these are sylvite, or potassium chloride (KCl); sylvinite, or sodium potassium chloride (NaCl KCl); carnallite, or potassium magnesium chloride (KCl MgCl2); langbeinite, or potassium magnesium sulfate (K2SO4 2MgSO4); and polyhalite, or calcium magnesium potassium sulfate (2CaSO4 MgSO4 K2SO4). Isotppes There are three naturally occurring isotopes of potassium, potassium-39, potassium-40, and potassium-41. Potassium-40 is radioactive. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope. About ten artificial radioactive isotopes of potassium are known also. A radioactive isotope is one that breaks apart and gives off some form of radiation. Artificially radioactive isotopes are produced when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive. Potassium-40 is of special interest to scientists. Potassium is widely distributed in nature in plants, animals, and rocks. That means that nearly everything on Earth contains at least a tiny amount of radioactive potassium-40. That includes the human body! About 0.012 percent of the potassium in the human body is radioactive potassium-40. However, that is not enough radiation to cause any harm. Radioactive potassium-40 in rocks can be used to measure the age of objects. When the isotope gives off radiation, it breaks down to an isotope of argon: A scientist can analyze a rock to see how much potassium-40 and how much argon-40 it contains. The older the rock, the more argon-40 and the less potassium-40 it contains. The younger the rock, the more potassium-40 and the less argon-40 it contains. One might wonder why argon gas does not escape into the atmosphere. The answer is that argon gas is trapped within the solid rock. It is released only when the potassium-dating process is conducted.
uses Potassium metal is sometimes used as a heat exchange medium. A heat exchange medium is a material that picks up heat in one place and carries it to another place. Potassium metal is sometimes used as a heat exchange medium in nuclear power plants. There, heat is produced at the core, or center, of the reactor. Liquid potassium is sealed into pipes surrounding the core. As heat is given off, it is absorbed (taken up) by the potassium. The potassium is then forced through the pipes into a nearby room. In that room, the potassium pipes are wrapped around pipes filled with water. The heat in the potassium warms the water. Eventually the water gets hot enough to boil. It changes into steam and is used to operate devices that generate electricity. By far the most important compound of potassium is potassium chloride (usually referred to as "potash," of course!). At least 85 percent of that compound is used to make synthetic (artificial) fertilizers. In 1996, about 1.5 billion kilograms (3.4 billion pounds or 1.7 million tons) of potassium chloride was produced in the United States for use in fertilizers. Many other potassium compounds are commercially important, although no use begins to compare with the amount of potash used for fertilizers. Some examples of other important potassium compounds are the following: Potassium is one of the three primary nutrients, or macronutrients, required by plants. potassium bicarbonate, or baking soda (KHCO3): baking powders; antacid (for upset stomach); food additive; soft drinks; fire extinguishers
potassium bisulfite (KHSO3): food preservative (but not in meats); bleaching of textiles and straw; wineand beer-making; tanning of leathers potassium bitartrate, or cream of tartar (KHC4H4O6): baking powder; "tinning" of metals; food additive potassium bromide (KBr): photographic film; engraving potassium carbonate, or potash (K2CO3): specialized glasses and soaps; food additive potassium chromate (K2CrO4): dyes and stains (bright yellowish-red color); explosives and fireworks; safety matches; tanning of leather; fly paper potassium fluorosilicate (K2SiF6): specialized glasses, ceramics, and enamels;insecticide potassium hydroxide, or caustic potash (KOH): paint remover; manufacture of specialized soaps; fuel cells and batteries; bleaching; food additive; herbicide potassium nitrate, or nitre, or saltpeter (KNO3): explosives, fireworks, matches, rocket fuel; manufacture of glass; curing of foods potassium pyrophosphate, or tetrapotassium pyrophosphate, or TKPP (K4P2O7): soaps and detergents potassium sodium tartrate, or Rochelle salt (KNaC4H4O6): baking powder; medicine; silvering of mirrors
Molecule weight of Rb =85.468 Atomic number =37 Reaction of rubidium with air Rubidium is very soft and easily cut. The resulting surface is bright and shiny. However, this surface soon tarnishes because of reaction with oxygen and moisture from the air. If rubidium is burned in air, the result is mainly formation of dark brown rubidium superoxide, RbO2. Rb(s) + O2(g) RbO2(s) Reaction of rubidium with water Rubidium metal reacts very rapidly with water to form a colourless solution of rubidium hydroxide (RbOH) and hydrogen gas (H2). The resulting solution is basic because of the dissolved hydroxide. The reaction is very exothermic. The reaction is so fast that if the reaction is carried out in a glass vessel, the glass container may well shatter. The reaction is slower than that of caesium (immediately below rubidium in the periodic table), but faster than that of potassium (immediately above rubidium in the periodic table). 2K(s) + 2H2O 2KOH(aq) + H2(g)
Reaction of rubidium with the halogens Rubidium metal reacts vigorously with all the halogens to form rubidium halides. So, it reacts with fluorine, F2, chlorine, Cl2, bromine, I2, and iodine, I2, to form respectively rubidium(I) bromide, RbF, rubidium(I) chloride, RbCl, rubidium(I) bromide, RbBr, and rubidium(I) iodide, RbI. 2Rb(s) + F2(g) RbF(s) 2Rb(s) + Cl2(g) RbCl(s) 2Rb(s) + Br2(g) RbBr(s) 2Rb(s) + I2(g) RbI(s) Reaction of rubidium with acids Rubidium metal dissolves readily in dilute sulphuric acid to form solutions containing the aquated Rb(I) ion together with hydrogen gas, H2. 2Rb(s) + H2SO4(aq) 2Rb+(aq) + SO42-(aq) + H2(g) Reaction of rubidium with bases Rubidium metal reacts very rapidly with water to form a colourless basic solution of rubidium hydroxide (RbOH) and hydrogen gas (H2). The reaction continues even when the solution becomes basic. The resulting solution is basic because of the dissolved hydroxide. The reaction is very exothermic. The reaction is so fast that if the reaction is carried out in a glass vessel, the glass container may well shatter. The reaction is slower than that of caesium (immediately below rubidium in the periodic table), but faster than that of potassium (immediately above rubidium in the periodic table). As the reaction proceeds, the concentration of base increases. 2K(s) + 2H2O 2KOH(aq) + H2(g)
Reaction of cesium with air Caesium (cesium in USA) is very soft and easily cut. The resulting surface is bright and shiny. However, this surface soon tarnishes because of reaction with oxygen and moisture from the air. If caesium is burned in air, the result is mainly formation of orange caesium superoxide, CsO2. Cs(s) + O2(g) CsO2(s) Reaction of caesium with water Caesium (cesium in USA) metal reacts rapidly with water to form a colourless solution of caesium hydroxide (CsOH) and hydrogen gas (H2). The resulting solution is basic because of the dissolved hydroxide. The reaction is very exothermic. The reaction is so fast that if the reaction is carried out in a glass vessel, the glass container will shatter. Although not known for certain, the reaction is probably slower than that of francium (immediately below caesium in the periodic table). The reaction is faster than that of rubidium (immediately above caesium in the periodic table). 2Cs(s) + 2H2O 2CsOH(aq) + H2(g) Reaction of caesium with the halogens
Reaction of cesium with acids Caesium (cesium in USA) metal dissolves readily in dilute sulphuric acid to form solutions containing the aquated Cs(I) ion together with hydrogen gas, H2. 2Cs(s) + H2SO4(aq) 2Cs+(aq) + SO42-(aq) + H2(g) Reaction of caesium with bases Caesium (cesium in USA) metal reacts rapidly with water to form a colourless basic solution of caesium hydroxide (CsOH) and hydrogen gas (H2). The reaction continues even when the solution becomes basic. The resulting solution is basic because of the dissolved hydroxide. The reaction is very exothermic. The reaction is so fast that if the reaction is carried out in a glass vessel, the glass container will shatter. Although not known for certain, the reaction is probably slower than that of francium (immediately below caesium in the periodic table). The reaction is faster than that of rubidium . As there action continues, the concentration of base increases. 2Cs(s) + 2H2O 2CsOH(aq) + H2(g)
Reaction of caesium with the halogens Cesium (cesium in USA) metal reacts vigorously with all the halogens to form caesium halides. So, it reacts with fluorine, F2, chlorine, Cl2, bromine, I2, and iodine, I2, to form respectively caesium(I) bromide, CsF, caesium(I) chloride, CsCl, caesium(I) bromide, CsBr, and caesium(I) iodide, CsI. 2Cs(s) + F2(g) CsF(s) 2Cs(s) + Cl2(g) CsCl(s) 2Cs(s) + Br2(g) CsBr(s) 2Cs(s) + I2(g) CsI(s) Reaction of cesium with acids Caesium (cesium in USA) metal dissolves readily in dilute sulphuric acid to form solutions containing the aquated Cs(I) ion together with hydrogen gas, H2. 2Cs(s) + H2SO4(aq) 2Cs+(aq) + SO42-(aq) + H2(g) Reaction of caesium with bases Caesium (cesium in USA) metal reacts rapidly with water to form a colourless basic solution of caesium hydroxide (CsOH) and hydrogen gas (H2). The reaction continues even when the solution becomes basic. The resulting solution is basic because of the dissolved hydroxide. The reaction is very exothermic. The reaction is so fast that if the reaction is carried out in a glass vessel, the glass container will shatter. Although not known for certain, the reaction is probably slower than that of francium (immediately below caesium in the periodic table). The reaction is faster than that of rubidium . As there action continues, the concentration of base increases. 2Cs(s) + 2H2O 2CsOH(aq) + H2(g)