Insights into the Periodic Law and Periodic Table
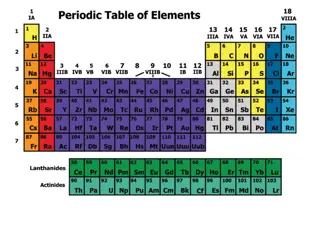
The Periodic Law dictates the systematic recurrence of physical and chemical properties of elements when arranged by increasing atomic number. This law, discovered in the 19th century by scientists like Lothar Meyer and Dmitri Mendeleev, is reflected in the Periodic Table where trends in properties such as atomic radius, ionization energy, electron affinity, and more become evident. Understanding these properties aids in predicting behaviors of elements based on their placement on the table.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Periodic Law and Periodic Table
Periodic Law Definition The Periodic Law states that the physical and chemical properties of the elements recur in a systematic and predictable way when the elements are arranged in order of increasing atomic number. Many of the properties recur at intervals. When the elements are arranged correctly, the trends in element properties become apparent and can be used to make predictions about unknown or unfamiliar elements, simply based on their placement on the table.
Discovery of Periodic Law Periodic Law was formulated based on observations made by scientists in the 19th century. In particular, contributions made by Lothar Meyer and Dmitri Mendeleev made trends in element properties apparent. They independently proposed Periodic Law in 1869. The periodic table arranged the elements to reflect Periodic Law, even though scientists at the time had no explanation for why properties followed a trend. Once the electronic structure of atoms was discovered and understood, it became apparent the reason characteristics occurred in intervals was because of the behavior of electron shells.
Properties Affected by Periodic Law The key properties that follow trends according to Periodic Law are atomic radius, ionic radius, ionization energy, electronegativity, and electron affinity. Atomic and ionic radius are a measure of the size of a single atom or ion. While atomic and ionic radius are different from each other, they follow the same general trend. The radius increases moving down an element group and generally decreases moving left to right across a period or row. Ionization energy is a measure of how easy it is to remove an electron from an atom or ion. This value decreases moving down a group and increases moving left to right across a period.
Properties Affected by Periodic Law Electron affinity is how easily an atom accepts an electron. Using Periodic Law, it becomes apparent the alkaline earth elements have a low electron affinity. In contrast, the halogens readily accept electrons to fill their electron subshells and have high electron affinities. The noble gas elements have practically zero electron affinity because they have full valence electron subshells. Electronegativity is related to electron affinity. It reflects how easily an atom of an element attracts electrons to form a chemical bond. Both electron affinity and electronegativity tend to decrease moving down a group and increase moving across a period. Electropositivity is another trend governed by Periodic Law. Electropositive elements have low electronegativities (e.g., cesium, francium). In addition to these properties, there are other characteristics associated with Periodic Law, which may be considered properties of element groups. For example, all of the elements in group I (alkali metals) are shiny, carry a +1 oxidation state, react with water, and occur in compounds rather than as free elements.
Chemical elements 1 H Hydrogen [ ha dr d n ] 21 Sc Scandium [ sk nd m ] 22 Ti 23 V 24 Cr 25 Mn 26 Fe 27 Co 28 Ni 29 Cu 30 Zn 31 Ga Titanium Vanadium Chromium Manganese Iron Cobalt Nickel Copper Zinc Gallium [ t(a) 'te n m ] [ v ne di m ] [ kr m m ] [ m ni z ] [ a n ] [ k b lt ] [ n k l ] [ k p ] [ z k ] [ li m ] 2 He 3 Li 4 Be 5 B 6 C 7 N 8 O 9 F 10 Ne 11 Na Helium Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon Sodium [ hi l m ] [ l i m ] [ b r li m ] [ b r n ] [ k b n ] [ na tr d n ] [ ks d n ] [ fl ri n ] [ ni n ] [ s d m ] 32 Ge Germanium [ d me ni m ] 12 Mg Magnesium [ m ni z m ] 33 As 34 Se 35 Br 36 Kr 37 Rb 38 Sr 39 Y 40 Zr Arsenic Selenium Bromine Krypton Rubidium Strontium Yttrium Zirconium [ sn k ] [ s li n m ] [ br mi n ] [ kr pt n ] [ r b di m ] [ str nt m ] [ tri m ] [ z k ni m ] 13 Al 14 Si 15 P 16 S 17 Cl 18 Ar 19 K 20 Ca Aluminum Silicon Phosphorus Sulfur Chlorine Argon Potassium Calcium [ lu m n m ] [ s l k n ] [ f sf r s ] [ s lf ] [ kl ri n ] [ n ] [ p t s m ] [ k ls m ]
Chemical elements 41 Nb Niobium [ na ' ub m ] 61 Pm Promethium [ pr mi i m ] 42 Mo 43 Tc 44 Ru 45 Rh 46 Pd 47 Ag 48 Cd 49 In 50 Sn 51 Sb Molybdenum Technetium Ruthenium Rhodium Palladium Silver Cadmium Indium Tin Antimony [ m l bd n m ] [ tek'ni m ] [ ru i nj m ] [ r di m ] [ p le di m ] [ s lv ] [ k dm m ] [ ndi m ] [ t n ] [ nt m ni ] 62 Sm 63 Eu 64 Gd 65 Tb 66 Dy 67 Ho 68 Er 69 Tm 70 Yb 71 Lu Samarium Europium Gadolinium Terbium Dysprosium Holmium Erbium Thulium Ytterbium Lutetium [ s me r m ] [ j ropi m ] [ d l n m ] [ t b m ] [ d s pr m ] [ holmi m ] [ bi m ] [ ju l m ] [ t bj m ] [ lu: ti: j m ] 52 Te Tellurium [ te l ri m ] 72 Hf Hafnium [ h fni m ] 53 I 54 Xe 55 Cs 56 Ba 57 La 58 Ce 59 Pr 60 Nd Iodine Xenon Cesium Barium Lanthanum Cerium Praseodymium Neodymium [ a di n ] [ zen n ] [ zi n n ] [ si zi m ] [ be ri m ] [ l n n m ] [ s r m ] [ pre zi d m m] [ ni u'd m m ] 73 Ta 74 W 75 Re 76 Os 77 Ir 78 Pt 79 Au 80 Hg Tantalum Tungsten Rhenium Osmium Iridium Platinum Gold Mercury [ t nt l m ] [ t st n ] [ ri ni m ] [ zmi m ] [ r d m ] [ pl t n m ] [ ld ] [ m kj ri ]
Chemical elements 81 Tl Thallium [ li m ] 101 Md Mendelevium [ mend li vi m ] 82 83 84 85 86 87 88 89 90 91 Pb Bi Po At Rn Fr Ra Ac Th Pa Protactinium [ pr t k t ni m ] Lead Bismuth Polonium Astatine Radon Francium Radium Actinium Thorium [ led ] [ b zm ] [ p l ni m ] [ ' st ti n ] [ re d n ] [ 'fr ns m ] [ re d m ] [ k t n m ] [ ri m ] 102 No Nobelium [ no beli m ] 103 Lr Lawrencium [ l rensi m ] 104 Rf Rutherfordium [ r 'f d m ] 105 Db Dubnium [ 'd bn m ] 106 Sg Seaborgium [ 'si b g m ] 107 Bh Bohrium [ 'b r m ] 108 Hs Hassium [ 'h s m ] 109 Mt Meitnerium [ ma t'n r m ] 110 Ds Darmstadtium 92 U Uranium [ j re n m ] 111 Rg Roentgenium 93 94 95 96 97 98 99 100 Fm Np Pu Am Cm Bk Cf Es Californium Einsteinium Neptunium Plutonium Americium Curium Berkelium [ nep tju ni m ] [ plu t n m ] [ m r si m ] [ kj r m ] [ b 'ki l m ] [ k l f nj m ] [ a n'sta n m ] [ fermi m ] 112 Cn Copernicium 113 Nh 114 Fl 115 Mc 116 Lv 117 Ts 118 Og Nihonium Flerovium Moscovium Livermorium Tennessine Oganesson Fermium
Pronunciation https://quizlet.com/124752264/chemical-elements- crossword-puzzle-flash-cards/