Exploring the Fundamental Concepts of the Periodic Table
Explore the foundational principles of the periodic table, tracing back to Dimitri Mendeleev's pioneering work in creating the first modern version. Unveil the significance of the columns, aptly named _________________, which structure the elements, alongside an elucidation of the rows that extend horizontally. Gain insight into the essence of the periodic table and its enduring legacy in the realm of chemistry.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
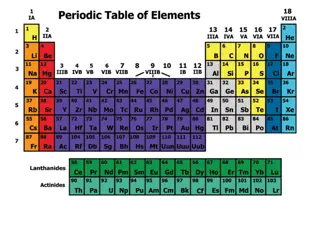
OB: Trends of the Periodic Table 1. Dimitri Mendeleev created the first modern periodic table, ours is modeled on it.
2 The COLUMNS on the table are called _________________, there are ________ on the table. 3 The ROWS that go across the table to the right are called ___________________, there are _____ of these. Look at your periodic table now, touch numbers 1-18 for the groups, then touch 1-7 for the periods.
4. Group 1 (Li to Fr) are called the ALKALI metals
5. Group 2 (Be to Ra) are called the ALKALINE EARTH metals
6. Group 3-12 plus under the staircase are called the TRANSITIONAL metals
7. To the right of the staircase, AND Hydrogen Are called the NONMETALS
8. Group 17 are called the HALOGENS they are also nonmetals
9. Group 18 are called the Noble Gases they are also nonmetals
10. seven atoms that touch the staircase are called the METALLOIDS NOT Al or Po
11. the 2 rows at the bottom of the table are called the INNER TRANSITIONAL metals. They fit inside group 3
12. Atoms in the same group share many chemical properties, because the have the same number of valence (or outermost) electrons. which means these atoms in the group will bond in similar ways. Examples: all make 1:1 compounds: LiCl, NaCl, and KCl All make 1:2 compounds: BeBr2, MgBr2, CaBr2
Whats up with the name of this table? 14. What does PERIODIC even mean here?
Whats up with the name of this table? 14. What does PERIODIC even mean here? Periodic refers to a pattern or normal repetition of things. Magazines are periodicals, they come periodically. Sports Illustrated comes weekly. National Geographic comes monthly. When I was a kid, there was a men s magazine called Gentlemen s Quarterly. It came 4x per year (once per quarter). Now it s GQ - and it comes 10X per year.
15. The elements of the Periodic Table are arranged in order of increasing atomic number. That means by increasing number of PROTONS.
16. The Periodic Law When the elements are arranged in order of increasing atomic number there is a periodic repetition of similar chemical properties - in the groups. This works because of the weird shape of the table.
17. Similar properties show up periodically, IN THE GROUPS. 18. At the top of the table, this happens every 8 atoms, but starting in the middle, it s every 18 atoms.
19. The periods of the table go left to right. 1 20. The periods contain many elements that have very different properties. Periods 2 3 4 5 6 7 21. Period numbers go 1 to 7. They correspond to the number of electron orbitals that all of the atoms in that period have. 6 7
22. The period number (1-7) is indicative of how many electron orbitals each atom in the period has. Number of electron orbitals period Example element Electron configuration 1 H 2 Be 3 S 4 Mn 5 Xe 6 Ba 7 Ra
22. The period number (1-7) is indicative of how many electron orbitals each atom in the period has. Number of electron orbitals 1 period Example element Electron configuration 1 1 H 2 Be 3 S 4 Mn 5 Xe 6 Ba 7 Ra
22. The period number (1-7) is indicative of how many electron orbitals each atom in the period has. Number of electron orbitals 1 2 period Example element Electron configuration 1 2-2 1 H 2 Be 3 S 4 Mn 5 Xe 6 Ba 7 Ra
22. The period number (1-7) is indicative of how many electron orbitals each atom in the period has. Number of electron orbitals 1 2 3 period Example element Electron configuration 1 2-2 2-8-6 1 H 2 Be 3 S 4 Mn 5 Xe 6 Ba 7 Ra
22. The period number (1-7) is indicative of how many electron orbitals each atom in the period has. Number of electron orbitals 1 2 3 4 period Example element Electron configuration 1 2-2 2-8-6 2-8-13-2 1 H 2 Be 3 S 4 Mn 5 Xe 6 Ba 7 Ra
22. The period number (1-7) is indicative of how many electron orbitals each atom in the period has. Number of electron orbitals 1 2 3 4 5 period Example element Electron configuration 1 2-2 2-8-6 2-8-13-2 2-8-18-18-8 1 H 2 Be 3 S 4 Mn 5 Xe 6 Ba 7 Ra
22. The period number (1-7) is indicative of how many electron orbitals each atom in the period has. Number of electron orbitals 1 2 3 4 5 6 period Example element Electron configuration 1 2-2 2-8-6 2-8-13-2 2-8-18-18-8 2-8-18-18-8-2 1 H 2 Be 3 S 4 Mn 5 Xe 6 Ba 7 Ra
22. The period number (1-7) is indicative of how many electron orbitals each atom in the period has. Number of electron orbitals 1 2 3 4 5 6 7 period Example element Electron configuration 1 2-2 2-8-6 2-8-13-2 2-8-18-18-8 2-8-18-18-8-2 2-8-18-32-18-8-2 1 H 2 Be 3 S 4 Mn 5 Xe 6 Ba 7 Ra
The numbers in the element boxes on periodic table help us know many things. Locate the carbon atom in the key (the big one at top) Find Atomic Mass, Atomic Number, Electron Configuration, and selected Oxidation Numbers. The mass is the mass of the protons and the neutrons in the nucleus 23. Each of these subatomic particles fits into this table, you should memorize this. Particle Location Charge Mass symbol Proton Neutron Electron
The numbers in the element boxes on periodic table help us know many things. Locate the carbon atom in the key (the big one at top) Find Atomic Mass, Atomic Number, Electron Configuration, and selected Oxidation Numbers. The mass is the mass of the protons and the neutrons in the nucleus 23. Each of these subatomic particles fits into this table, you should memorize this. Particle Location Charge Mass symbol Proton Nucleus +1 1 amu p+ Neutron Electron
The numbers in the element boxes on periodic table help us know many things. Locate the carbon atom in the key (the big one at top) Find Atomic Mass, Atomic Number, Electron Configuration, and selected Oxidation Numbers. The mass is the mass of the protons and the neutrons in the nucleus 23. Each of these subatomic particles fits into this table, you should memorize this. Particle Location Charge Mass symbol Proton Nucleus +1 1 amu p+ Neutron Nucleus none 1 amu n Electron
The numbers in the element boxes on periodic table help us know many things. Locate the carbon atom in the key (the big one at top) Find Atomic Mass, Atomic Number, Electron Configuration, and selected Oxidation Numbers. The mass is the mass of the protons and the neutrons in the nucleus 23. Each of these subatomic particles fits into this table, you should memorize this. Particle Location Charge Mass symbol Proton Nucleus +1 1 amu p+ Neutron Nucleus none 1 amu n Outside the nucleus Electron Zero in H.S. 1 e
Another way to write this information (for cobalt) would be this: Co 27 59 This shows the average mass of cobalt to be 59 amu, and that it has an atomic number of 27. 24. Calculate the number of protons, neutrons and electrons for this element now.
Co 59 27 Mass = total number of p+ and n = 59 minus atomic number = p+ -27 Gives us the left over neutrons = 32 The atomic number is the number of protons, which is the same as the number of electrons (the positives = the negatives). 24. Cobalt has 27 protons, 27 electrons, and 32 neutrons.
25. List ALL of the nonmetals (by symbol, in numeric order) H, He B, C, N, O, F, Ne Si, P, S, Cl, Ar, Count them, there are 22 Non metals As, Se, Br, Kr Te, I, Xe, At, Rn
26. List all of the metalloids by symbol and name Count them, there are 7 Metalloids B for boron (NM) Si for silicon (NM) Ge for germanium (NM) As for arsenic (NM) Sb for antimony (M) Te for tellurium (M) At for astatine (NM) Two other METALS touch the staircase, Al and Po (the dog food exception to this trend)
27. How many elements are METALS? We won t list them all. All elements are metals except the nonmetals. There are 118 elements 22 nonmetals = 96 metals
Start Class #2 here #28 - #42
28. There are seven trends that we examine as group trends (patterns going down a group), or period trends (patterns going across the table). 29. These trends are: atomic radius (size), cation + anion sizes, net nuclear charge, electronegativity value, atomic mass, metallic and nonmetallic properties, and 1st ionization energy.
30. What is the group trend for atomic mass? Mass in amu Atom To figure it out we need to look at a group, and write out the atomic masses in order to see if there is a pattern. Use group 2. Fill in the chart now. Then we will describe this with as few words as possible. Be Mg Ca Sr
30. What is the group trend for atomic mass? Mass in amu 9 24 40 88 Atom The group trend for atomic mass is increasing. Be Mg Ca Sr
31. What is period trend for atomic mass. Atom Mass in amu Na Mg Al Si
31. What is period trend for atomic mass. The period trend for atomic mass is increasing. Atom Mass in amu Na 23 Mg 24 Al Si 27 28
32. What is the group trend for atomic radius (size)? To figure it out we need to look at a group, and what the pattern is. Use group 1. Fill in this chart. Then we will describe the trend with as few words as possible. RADIUS in pm Atom Li Na K Rb
32. What is the group trend for atomic radius (size)? The group trend for atomic radius is INCREASING. RADIUS in pm Atom 130. Li 160. Na 200. What s going on with this trend? Why does this trend exist? K 215 Rb
32. What is the group trend for atomic radius (size)? The group trend for atomic radius is INCREASING. RADIUS in pm Atom Li 130. What s going on with this trend? Why does this trend exist? Each period below has ONE MORE ELECTRON ORBITAL than the atom above it, so yes, this is sensible. Na 160. K 200. Rb 215
33. What is the period trend for atomic radius (size)? To figure it out we need to look at a period, write out the atomic radii - in order, to see what the pattern is. Use period 2. Fill in this chart now, then use as few words as possible to state the trend. Atom Li Be B C Mass in amu
33. What is the period trend for atomic radius (size)? To figure it out we need to look at a period, write out the atomic radii - in order, to see what the pattern is. Use period 2. Fill in this chart now, then use as few words as possible to state the trend. Atom Li Be B C 130. 99 84 75 Mass in amu
33. What is the period trend for atomic radius (size)? To figure it out we need to look at a period, write out the atomic radii - in order, to see what the pattern is. Use period 2. Fill in this chart now, then use as few words as possible to state the trend. The period trend for atomic radius is decreasing. How is this possible? The reason for this is a little more complicated. Atom Li Be B C 130. 99 84 75 Mass in amu
The period trend for atomic radius is decreasing. How is this possible? The reason for this is a little more complicated. All atoms in period 2 have 2 orbitals. As we move across the table, the number of orbitals is constant, but the number of protons in the center of the atom in the nucleus increases. More protons means more positive charge in the nucleus. This creates a stronger and stronger inward attraction, which pulls the atoms smaller, they shrink as we move to the right. The smallest atom in the period has the most protons the noble gas.