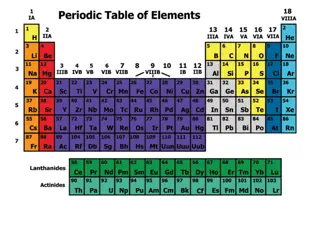
Understanding the Periodic Table and Chemical Bonds in Physical Science
The periodic table organizes elements based on their properties, with rows representing periods and columns representing groups. Mendeleev's early table laid the foundation for predicting undiscovered elements. Today's periodic table orders elements by atomic number, showcasing the periodic law and
3 views • 15 slides
Understanding the Periodic Table: From Mendeleev to Modern Classification
Organizing elements based on their properties led to the development of the periodic table by Mendeleev with predictions that have stood the test of time. The modern periodic table arranges elements by increasing atomic number, showcasing repeating patterns of properties. It categorizes elements int
1 views • 29 slides
Classification of Elements and Periodicity in Properties: Overview and Evolution
The journey of understanding the classification of elements and periodicity in properties begins with early laws like the Law of Triads and Newland's Law of Octaves. Mendeleev's Periodic Law revolutionized the organization of elements, leading to the modern periodic table. Discoveries of eka-alumini
3 views • 32 slides
Evolution of Periodic Table and Classification of Elements
The Periodic Table is a systematic arrangement of elements based on atomic number and properties. Over time, chemists developed various classification methods such as Dobereiner's Triads, Newland's Law of Octaves, Mendeleev's Periodic Table, and the Modern Periodic Table to organize the increasing n
1 views • 26 slides
The History and Significance of the Modern Periodic Table
Chemists in the nineteenth century categorized elements based on similarities in properties, leading to the development of the modern periodic table. Dmitri Mendeleev and Lothar Meyer played key roles in organizing elements by atomic mass and predicting unknown elements. Mendeleev's accurate predict
0 views • 17 slides
Understanding the Periodic Table: Atomic Structure and Element Classification
Explore the fundamental concepts of the periodic table, including how to locate atomic numbers and masses, identify element groups, understand group and period distinctions, and analyze element properties. Delve into historical perspectives from scientists like D. Bereiner and Mendeleev, and grasp t
0 views • 38 slides
Insights into the Periodic Law and Periodic Table
The Periodic Law dictates the systematic recurrence of physical and chemical properties of elements when arranged by increasing atomic number. This law, discovered in the 19th century by scientists like Lothar Meyer and Dmitri Mendeleev, is reflected in the Periodic Table where trends in properties
0 views • 12 slides
Understanding the Periodic Table and Valence Electrons
Dmitri Mendeleev's organization of elements into the periodic table, the layout of periods and groups, and the significance of valence electrons in determining chemical reactivity are explored in this informative content. Discover how valence electrons relate to group numbers and learn about excepti
0 views • 31 slides
Exploring Atoms, Elements, and the Periodic Table
Delve into the fascinating world of atoms and the periodic table with experiments on magnesium and zinc oxides. Discover how different elements combine to form compounds and analyze their composition. Learn about the relative atomic weights according to historical figures like John Dalton and Amadeo
0 views • 17 slides
Understanding the Periodic Table: Mendeleev's Discovery and the Role of Valence Electrons
Russian chemist Dmitri Mendeleev's groundbreaking work in 1869 led to the creation of the periodic table, showcasing a pattern in the arrangement of elements by increasing atomic mass. Discover how Mendeleev's table evolved, the significance of Henry Moseley's contribution in 1914, and the importanc
0 views • 61 slides
Exploring the Fundamental Concepts of the Periodic Table
Explore the foundational principles of the periodic table, tracing back to Dimitri Mendeleev's pioneering work in creating the first modern version. Unveil the significance of the columns, aptly named _________________, which structure the elements, alongside an elucidation of the rows that extend h
0 views • 140 slides
Evolution of the Periodic Table: From Dobereiner to Mendeleev
The evolution of the periodic table, from Dobereiner's Law of Triads to Newlands' Law of Octave, and finally Mendeleev's Periodic Law and Original Periodic Table, marked significant strides in organizing chemical elements based on their properties and atomic weights. Each contribution led to the ref
0 views • 14 slides
Understanding Chemistry: Elements, Periodic Table, and Interactions
Delve into the world of chemistry, exploring the fundamental concepts of elements, the development of the periodic table by scientists like Mendeleev and Moseley, and how elements interact with each other. Discover the properties of elements, atomic structures, and the organization of the periodic t
0 views • 31 slides
Exploring the Periodic Table: Historical Development and Elemental Properties
Delve into the evolution of the periodic table from Mendeleev to Moseley, understanding how elements are arranged based on atomic number to reveal periodic trends. Learn about the significance of groups, diatomic elements, and valence electrons in defining the properties of elements.
0 views • 40 slides
Understanding the Classification of Elements in the Periodic Table
In 1869, Mendeleev and Lothar Meyer published classification schemes for elements, leading to the development of the Periodic Table. This table organizes elements based on properties and reactivities, with each element having a unique atomic number. There are 92 naturally occurring and 13 man-made e
0 views • 31 slides
Understanding the Periodic Table: Elements, Trends, and Atomic Radius
The periodic table organizes elements by properties, with Mendeleev's contributions and predictions. Elements are grouped into periods and groups, displaying trends in ionization energy, electronegativity, and atomic size. Learn about the atomic radius, how it varies across periods and groups, influ
0 views • 20 slides