Understanding the Four States of Matter and Changes in States
Explore the four states of matter - solid, liquid, gas, and plasma - and understand their unique characteristics based on the attractions of their particles. Learn how heat energy can cause transitions between these states, such as melting, freezing, vaporization, condensation, and sublimation.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Chapter 9 Chapter 9 Heat and States of Matter Heat and States of Matter Section 2 States of Matter 1
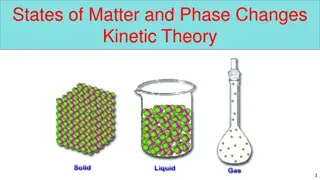
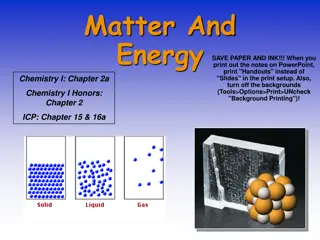
Four States of Matter Four States of Matter The four states of matter include: Solid Liquid Gas Plasma The differences between the four states are due to differences in the attractions of their particles.
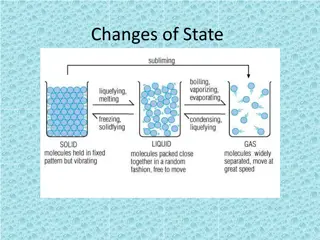
Four States of Matter Four States of Matter 1 Solid Particles are packed closely together Particles are constantly vibrating in place Attractions between particles is very strong Have a fixed volume and shape 2 Liquid Particles can slide past each other allowing the liquid to flow and take the shape of its container Attractions between particles are strong but not as strong as in solids; particles cling together Have a fixed volume but not a fixed shape 3 Gas Particles are much farther apart than liquids and solids Attraction between particles is weak; particles no longer cling together Have no definite shape or volume; particles spread out until evenly distributed (diffusion)
Four States of Matter Four States of Matter 4 Plasma Matter consisting of positively and negatively charged particles The most common state of matter in the universe Does not have a definite shape or volume Examples of plasmas include: Lightning Stars Neon and fluorescent tubes Auroras
Changing States Changing States Changes in thermal energy of a material can cause it to change from one state to another. Melting When energy and temperature increase causing a solid to turn into a liquid. The particles of the solid move faster and the attractive forces between them weaken. Freezing - When a liquid loses/decreases its energy and temperature causing the particles to move slower and strengthen its attractive forces and turns into a solid. Vaporization When a liquid gains/increases its energy and temperature causing the particles to move faster and the particles to no longer cling together and turn into a gas. Evaporation Vaporization on the surface of a liquid Boiling Vaporization throughout an entire liquid.
Changing States Changing States Condensation When a gas turns into a liquid due to a loss/decrease of energy and temperature; the kinetic energy decreases and the particles cling back to each other. Sublimation When a solid skips the liquid states and turns directly into a gas because of an increase in thermal energy and temperature.
Changing States Changing States