Understanding the Classification of Elements in the Periodic Table
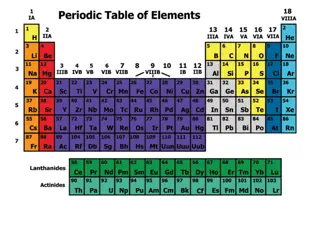
In 1869, Mendeleev and Lothar Meyer published classification schemes for elements, leading to the development of the Periodic Table. This table organizes elements based on properties and reactivities, with each element having a unique atomic number. There are 92 naturally occurring and 13 man-made elements, categorized into metals, nonmetals, and metalloids based on physical properties. Metals exhibit high luster, malleability, and conductivity, while nonmetals are dull and non-conductive. Metalloids have properties between metals and nonmetals. The Alkali Metals in Group IA are highly reactive due to having one electron in their outer shell.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Topic CLASSIFICATION OF ELEMENTS Presented by Dr. M. N. Naik Professor & Head Department of Environment science Deogiri College, Aurangabad
In 1869 Mendeleev and Lothar Meyer (Germany) published nearly identical classification schemes for elements known to date. The periodic table is base on the similarity of properties and reactivities exhibited by certain elements. Dmitri Mendeleev is known as the father of the Periodic Table. Later, Henri Moseley ( England,1887-1915) established that each elements has a unique atomic number, which is how the current periodic table is organized.
There are 92 naturally occurring and 13 man- made elements listed on the Periodic Table, with space left for future creations. These elements can be divided into three basic types, according to physical properties: metals, nonmetals, and metalloids. their chemical and
Metals: Most of the elements are metals. Characteristics of metals include: high luster, high malleability, high ductility, good conductivity of heat and electricity, and have high melting and boiling points. Metals tend to lose electrons in chemical bonding, forming positive ions. Metals are found on the left side of the Periodic Table.
Non metals: Nonmetals are found on the right side of the Periodic Table. Nonmetals are generally : dull, brittle, non-malleable, non-ductile, with low melting and boiling points. Many are gases at room temperature. They are non-conductors of heat and electricity. Nonmetals form negative ions because they tend to gain electrons during chemical bonding.
Metalloids : Metalloids have some of the properties of metals and some properties of nonmetals. They are found nonmetals in the Periodic Table. between metals and
The Alkali Metals Group IA : The Alkali Metals are found in the first group of the Periodic Table. They are a group of elements that are so reactive that they are always found combined with other elements in nature. H Li Na The reason that these elements are so reactive is that they only have one electron in their outer shell, which they lose when bonding with other elements, to get a full outer shell of electrons. The alkali metals form +1 ions. K Rb Cs Alkali Metals are malleable and ductile, good conductors of heat and electricity, but are very soft. Fr
The alkali metals include Lithium, Sodium, Potassium, Rubidium, Cesium, and Francium. Alkali metals can explode when in contact with water. Cesium and Francium are the most reactive in this group. Francium is the most reactive of all metals. Although Hydrogen is a nonmetal, it is often listed with this group because it has one electron in its only energy level. Hydrogen is highly reactive but only forms covalent bonds.
The Alkaline Earth Metals : Be The Alkaline Earth Elements are found in the II and IIA group of the Periodic Table. Mg Even though they are not as reactive as the alkali metals, the alkaline earth metals are highly reactive, and are not found in the elemental state in nature. Ca Sr Ba All these elements except Be have pronounced metallic properties. Ra
Each of the alkaline earth metals has two electrons in its outer energy level. Alkaline earth metals lose their two valence electrons and form +2 ions when bonding. In the free state they are grayish white substances harder than the alkali metals and have higher melting and boiling points. Alkaline earth metals are extremely electropositive and forms divalent ionic salts.
The oxides of calcium , strontium, barium combine directly with water to form hydroxides. Solubility of hydroxides increases from calcium to radium . Some salts are sparingly soluble in water , e.g. Carbonates, Sulphates and Phosphates. The alkaline earth elements include Beryllium, Magnesium, Calcium, Strontium, Barium, and Radium.
The Boron Family: The Boron Family is the third group on the Periodic Table. The members of this family have three electrons in their outer shell. B Al Although uncombined in nature, some of the other members of this family sometimes are. Boron is never found Ga In Members of the Boron Family tend to lose three electrons and form +3 ions. Tl
Boron is a metalloid, and all other group members are metals. Aluminum is found in this group. It is the most abundant metal on Earth. It is generally found in nature as aluminum oxide. Other elements found in this family are Gallium, Indium, and Thallium. Although Boron is a metalloid, all other elements in this family are metals.
The Carbon Family: C The Carbon family is the IV and IVA group, and contains one nonmetal, two metalloids, and two metals. Si All members of the carbon family have four electrons in their outer energy level. Members of this family include Carbon, Silicon, Germanium, Tin, and Lead. Members of this family tend to form covalent bonds. Ge Sn Pb Carbon is the Sixth most abundant element in the universe, but not as common on Earth.
Carbon exists in several bonding forms such as graphite and diamonds. C-14 is used in radiological dating of some fossils. It can form four covalent bonds with other elements and even other carbon atoms, resulting in an almost infinite number of carbon-containing compounds. Life on earth would be impossible without carbon. Silicon is also found in the Carbon Family. It is the second most abundant element in the Earth s crust, and is used in solar-electric cells and semi-conductors. Germanium is also used in electronic devices. Tin and lead have a number of uses.
The Nitrogen Family: The Nitrogen Family is the fifth group and contains two nonmetals, two metalloids, and only one metal. They all have five electrons in their outer energy level. N P They tend to gain three electrons when forming ionic bonds, and form -3 ions. As Nitrogen makes up about 4/5 of the Earth s atmosphere, and is a colorless, odorless gas at room temperature. It is a nonmetal. Sb Nitrogen forms many compounds with oxygen, such as NO2 and N2O. Bi
Phosphorus occurs naturally in solid form as red phosphorus or white phosphorus. It is highly reactive and will burst into flame in the presence of air. It is stored under water. Arsenic is a metalloid, and is used in the semiconductor industry and in manufacturing. It is highly poisonous and is used in weed killer and rat poison. Antimony is a hard brittle metalloid used in electronics. It is, like arsenic, highly poisonous. Bismuth is the heaviest naturally occurring element that is not radioactive. It has a variety of uses from Pepto- Bismol to paint pigments, to electrical solder and the heads of fire sprinkler systems.
The Oxygen Family : O Members of the Oxygen Family all have six electrons in their outermost energy level. S The Oxygen Family is the sixth group on the Periodic Table and contains three nonmetals and two metalloids. There are no metals in the Oxygen Family. Members of the Oxygen Family tend to gain two electrons and form -2 ions, when bonding ionically. Se Te Oxygen is the most common element in the Earth s crust and makes up 1/5 of the Earth s atmosphere. Po
It is a colorless, odorless gas at room temperature. All elements except the noble gases can form compounds with oxygen. Sulfur is a yellow solid at room temperature. Sulfur is in sulfuric acid and also in acid rain. It is in the gas H2S, which smells like rotten eggs. Selenium is a semiconductor that is sensitive to light, so it is often used in light sensors. Tellurium is a brittle metalloid used in blasting caps. Polonium is radioactive and was discovered by Marie Curie. It has few commercial uses.
The Halogens : All members of the Halogen Group have seven electrons in their outer energy level. F They are the seventh group on the Periodic Table. Cl This group contains four nonmetals and one metalloid. There are no metals among the halogens. Br I Because they only need one electron to complete their outer energy level, they are the most reactive of the nonmetals. They gain one electron and form -1 ions when bonding ionically. At
Fluorine is the most reactive nonmetal and is never found alone in nature. It is a poisonous greenish-yellow gas at room temperature. Fluorine compounds are added to many water systems because they prevent tooth decay. Teflon is a fluorocarbon compound. Chlorine is not as reactive as fluorine, but is also a reactive poisonous greenish-yellow gas. One of its most common compounds is NaCl, table salt.
Bromine is a reddish-brown liquid at room temperature. It is often used to disinfect hot tubs. Iodine is a solid at room temperature that turns to a purple gas when heated. It is needed in your diet to keep your thyroid gland functioning properly. Astatine is a radioactive metalloid, but not much is known about its properties
The Noble Gases : He The noble gases are the eighth group on the Periodic Table, and all of the members of this group are unreactive because their outer electron shell is already full. Ne Ar Helium is the second lightest and second most abundant gas in the universe, but is relatively rare on Earth. Kr Helium has two electrons in its only energy level. Xe No compounds of Helium are known. It is used in balloons and blimps. Rn
Neon is the best known of the noble gases because of its use in decorative lighting fixtures. It forms no compounds. Argon is used to fill incandescent light bulbs to keep the filament from burning up. It is also used in welding. Krypton is not a green solid that hurts Superman. It is an unreactive gas used in bright strobe lights and airport runway lights. Xenon is a gas that is also used in strobe lights. It can be forced to react with fluorine, but is ordinarily unreactive. Radon is a glowing yellow radioactive noble gas that is considered to be a health hazard if breathed in large amounts. It is used in cancer treatments.
The Transition Metals : Sc Ti V Cr Mn Fe Co Ni Cu Zn Y Zr Nb Mo Tc Ru Rb Pd Ag Cd La Hf Ta W Re Os Ir Pt Au Hg Ac Rf Db Sg Bh Hs Mt Uun Uuu Uub The Transition Metals are the elements found on the Periodic Table between the Alkaline Earth Metals and the Boron Family.
While the Transition Metals all have no more than 2 electrons in their outer energy level, their next energy level is incompletely filled. The properties of the Transition Metals depend on the electron configuration of the outer two energy levels. Transition elements form alloys easily. A copper-tin alloy is for mirrors and copper-zinc makes brass. Except for copper, the transition metals are all shiny and white, with high melting points and high densities. Many of the most commonly known metals, such as gold, silver, mercury, iron, copper and nickel are in this group.
The Rare Earth Elements : Lanthanide Series La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Actinide Series Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr
There are 30 Rare Earth Elements. They are all metals, and they are divided into two sets: The Lanthanide Series begins with element # 57, Lanthanum and ends with element # 71 Lutetium. The Actinide Series begins with element # 89 Actinium and ends with element # 103 Lawrencium. One element of the Lanthanide Series and most of the elements of the Actinide series are man-made. Many are radioactive. All of the Rare Earth elements are found in Group 3 of the Periodic Table in the sixth and seventh periods.