Creative Periodic Table Arrangement Techniques Explained
Discover unique ways to arrange the periodic table for better readability and understanding. Explore typewriter, normal, long, and spiral arrangements, along with an innovative Alexander Arrangement that connects the traditional table to a 3D model. Learn step-by-step instructions on how to cut, roll, and tape the elements to create a visual representation that enhances the structure of the elements.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
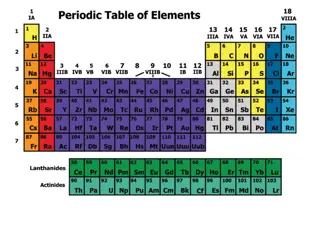
Periodic Table Arrangement
Typewriter arrangement The periodic table is made to fit on a piece of paper so it can be easily read and printed. However this leaves several gaps that can break the flow of the periodic table in the reader s eyes.
Long Arrangement Lanthanides and Actinides are supposed to be in there like this but it makes it less printable and readable. It also adds a huge disconnect between Beryllium and Boron (and others).
Alexander Arrangement of the Elements This connects the normal periodic table to the spiral arrangement by making a 3 dimensional model. All it takes is some scissors and tape.
How to Roll the pieces you have face out around a pen or pencil so that it coils easily when you release it. Fold creases on the edges of the transition metals marked by the dotted line
Now Tape the lanthanides and actinides in a loop coming out of the transition elements where they belong. Here
Cont. Push the transition metals so that they form a loop face out and tape group 2 next to group 13. In this picture the transition metals would be a loop flopping out of the screen. H Li Be Na Mg K Ca Transition metals Rb Sr Cs Ba Fr Ra
AAE Now fold the Noble Gases around so that they connect to the Alkali Metals. Connect He to Li (2-3) Ne to Na (10-11) and so forth Here is an overhead view of what you should have Main group elements Transition metals Lanthanide and actinide series