Understanding Moles and Formula Mass in Chemistry
Dive into the world of moles, Avogadro's number, and formula mass calculations in chemistry. Learn how to convert moles to grams, grams to moles, and determine the number of atoms using Avogadro's number. Explore the concepts of formula mass and percentage composition to understand the composition of compounds like magnesium carbonate. Unravel the difference between empirical and molecular formulas, essential in understanding the atomic composition of compounds.
Uploaded on Jul 29, 2024 | 0 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
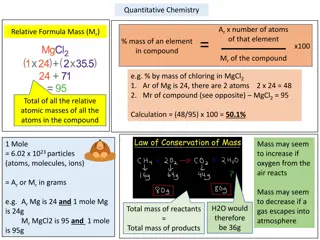
The Mole 1 dozen = 1 gross = 1 ream = 1 mole = 12 144 500 6.022 x 1023 There are exactly 12 grams of carbon-12 in one mole of carbon-12.
Avogadros Number 6.022 x 1023is called Avogadro s Number in honor of the Italian chemist Amadeo Avogadro (1776-1855). I didn t discover it. Its just named after me! Amadeo Avogadro
Calculations with Moles: Converting moles to grams How many grams of lithium are in 3.50 moles of lithium? 3.50 mol Li 6.94 g Li = g Li 45.1 1 mol Li
Calculations with Moles: Converting grams to moles How many moles of lithium are in 18.2 grams of lithium? 18.2 g Li 1 mol Li 2.62 = mol Li 6.94 g Li
Calculations with Moles: Using Avogadro s Number How many atoms of lithium are in 3.50 moles of lithium? 6.02 x 1023 atoms 3.50 mol = atoms 2.07 x 1024 1 mol
Calculations with Moles: Using Avogadro s Number How many atoms of lithium are in 18.2 g of lithium? 18.2 g Li 6.022 x 1023 atoms Li 1 mol Li 1 mol Li 6.94 g Li (18.2)(6.022 x 1023)/6.94 = atoms Li 1.58 x 1024
Calculating Formula Mass Calculate the formula mass of magnesium carbonate, MgCO3. 24.31 g + 12.01 g + 3(16.00 g) = 84.32 g
Calculating Percentage Composition Calculate the percentage composition of magnesium carbonate, MgCO3. From previous slide: 24.31 g + 12.01 g + 3(16.00 g) = 84.32 g 24.31 84.32 84.32 84.32 = 12.01 48.00 = 100 100 14.24% = 100 56.93% = 28.83% Mg C O 100.00 = =
Formulas Empirical formula: the lowest whole number ratio of atoms in a compound. Molecular formula: the true number of atoms of each element in the formula of a compound. molecular formula = (empirical formula)n [n = integer] molecular formula = C6H6 = (CH)6 empirical formula = CH
Formulas (continued) Formulas for ionic compounds are ALWAYS empirical (lowest whole number ratio). Examples: NaCl MgCl2 Al2(SO4)3 K2CO3
Formulas (continued) Formulas for molecular compounds MIGHT be empirical (lowest whole number ratio). Molecular: C6H12O6 H2O C12H22O11 Empirical: H2O CH2O C12H22O11
Empirical Formula Determination 1. Base calculation on 100 grams of compound. 2. Determine moles of each element in 100 grams of compound. 3. Divide each value of moles by the smallest of the values. 4. Multiply each number by an integer to obtain all whole numbers.
Empirical Formula Determination ( ( ( Adipic acid contains 49.32% C, 43.84% O, and 6.85% H by mass. What is the empirical formula of adipic acid? )( ( ( ( ) ) 49.32g C 1 mol C )( 6.85 g H 43.84 g O )( ) 1 mol H 1 mol O =4.107 mol C 6.78 = ) ) ) mol H mol O = 2.74 12.01 g C 1.01 16.00 g H g O
Empirical Formula Determination (part 2) Divide each value of moles by the smallest of the values. Carbon: Hydrogen: Oxygen:
Empirical Formula Determination (part 3) Multiply each number by an integer to obtain all whole numbers. Carbon: 1.50 Hydrogen: 2.50 Oxygen: 1.00 x 2 x 2 x 2 3 5 2 Empirical formula: C3H5O2
Finding the Molecular Formula The empirical formula for adipic acid is C3H5O2. The molecular mass of adipic acid is 146 g/mol. What is the molecular formula of adipic acid? 1. Find the formula mass of C3H5O2 3(12.01 g) + 5(1.01) + 2(16.00) = 73.08 g
Finding the Molecular Formula The empirical formula for adipic acid is C3H5O2. The molecular mass of adipic acid is 146 g/mol. What is the molecular formula of adipic acid? 2. Divide the molecular mass by the mass given by the emipirical formula. 146 73 3(12.01 g) + 5(1.01) + 2(16.00) = 73.08 g = 2
Finding the Molecular Formula The empirical formula for adipic acid is C3H5O2. The molecular mass of adipic acid is 146 g/mol. What is the molecular formula of adipic acid? 3. Multiply the empirical formula by this number to get the molecular formula. 146 73 3(12.01 g) + 5(1.01) + 2(16.00) = 73.08 g (C3H5O2) x 2 =C6H10O4 = 2