Converting Moles to Grams, Liters, Molecules, and Atoms
Explore the conversion factors for feet to inches and yards, and learn how to convert moles to grams, liters, molecules, and atoms using formula masses. Practice conversions and understand the relationship between moles, grams, and liters with helpful hints and examples.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
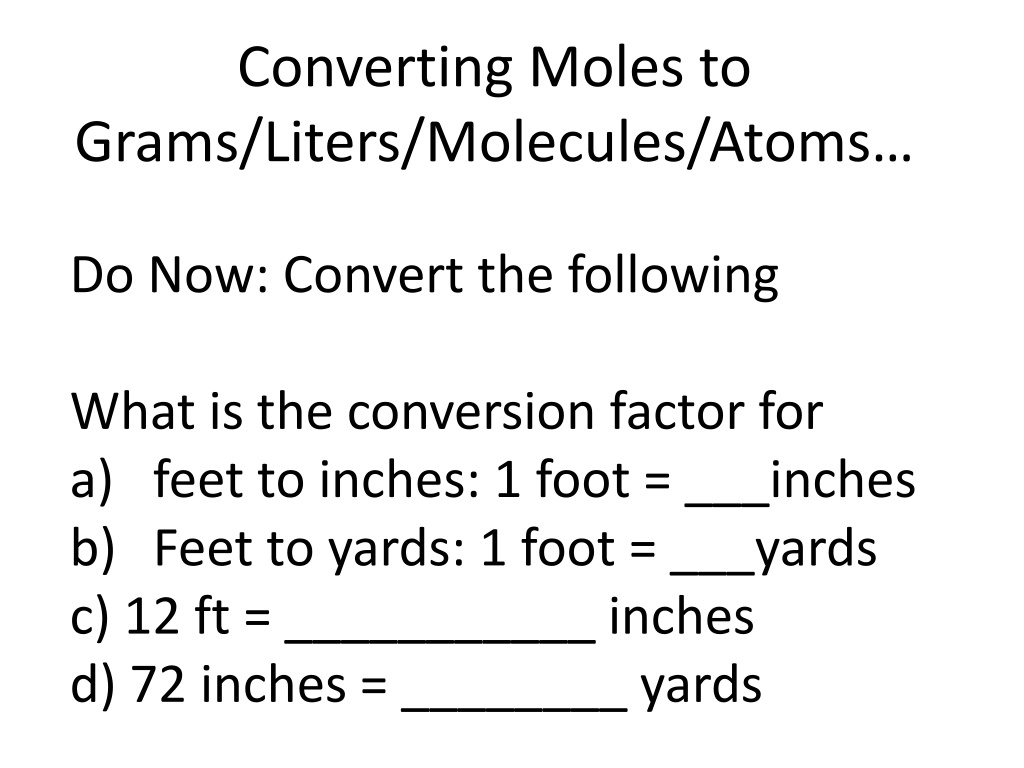
Converting Moles to Grams/Liters/Molecules/Atoms Do Now: Convert the following What is the conversion factor for a) feet to inches: 1 foot = ___inches b) Feet to yards: 1 foot = ___yards c) 12 ft = ___________ inches d) 72 inches = ________ yards
Atomic Mass Units are too small to measure on a laboratory balance, but grams are not. Thus, we use gram formula mass (formula mass) when converting between atoms/molecules, moles and grams packet p.8
2 3 no Convert to g 2 56 1 56 56 1 Conversion factor 112 g 1 mol= 56 g
Converting moles to grams (or grams to moles) HINT: when finding mass(grams) you have to calculate formula mass first Conversion factor is 1 mol = formula mass the set up is Given goes here (don t forget Unit) The unit you want to convert to goes here Conversion factor goes here (this unit must be the same as the unit in your given number) Nothing goes here
Now turn to p. 11 1 40 40 2 35 70 390 1 110 110 Conversion factor 3.54 mol 1 mol = 110g
You try p. 10 and 11 Here is something that can help Grams Formula Mass X Moles
Converting moles to liters (or liters to moles) Conversion factor is 1 mol = 22.4 L the set up is Given goes here (don t forget Unit) The unit you want to convert to goes here Conversion factor goes here (this unit must be the same as the unit in your given number) Nothing goes here
22.4 3.2 71.68L 1 Conversion factor 1mol = 22.4L
Here is something that can help Liters 22.4 X Moles
Converting moles to particles (or particles to moles) Hint: particles can be atoms, molecules, etc. Conversion factor is 1 mol = 6.02 x 1023 the set up is Given goes here (don t forget Unit) The unit you want to convert to goes here Conversion factor goes here (this unit must be the same as the unit in your given number) Nothing goes here
6.02 x 1023 3 1 18.03 x 1023 = 1.803 x 1024 Conversion factor 1 mol = 6.02 x 1023 particles
You try p. 9 and 14 Here is something that can help Liters 22.4 X X Moles 6.02 x 1023 Particles
Conversion Factors 1 mole = formula mass 1 mole = 22.4 L 1 mole = 6.02 x 1023 Given goes here (don t forget Unit)
TRICK moles to something (grams, liters, particles) MULTIPLY something (grams, liters, particles) to moles DIVIDE Grams 1 mole = formula mass 1 mole = 22.4 L 1 mole = 6.02 x 1023 FM X X Moles X Particles Liters
1 2.5 L 1 Conversion Factors used: 1 mol = FM and 1 mol = 22.4L
FOR WATER 1 g = 1 ml