Understanding Matter and Its Changes
Matter is anything that has mass and takes up space. By identifying physical properties and understanding physical changes, we can explore the different states of matter - solid, liquid, gas, and plasma. Measuring properties like length, mass, volume, and density helps us characterize and differentiate various forms of matter.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Matter and Its Changes Chapter Three
Matter is anything that has mass and takes up space.
We were able to identify these forms of matter because of their physical properties. Physical properties can be observed and measured without changing the properties of the matter.
A physical change causes a change in the matter but its identity stays the same.
Two physical properties that are often measured are length and mass.
If we multiply the length x width x heighth of a cube, we get its volume in cubic units.
If we divide mass by volume: g/cm3 We get density, a very important physical property.
Another important physical property is the state of matter.
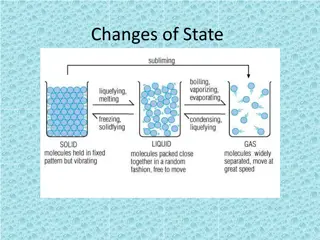
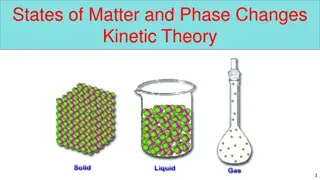
Matter has four states: Solid Liquid Gas Plasma
A solid has definite shape and volume because it has become frozen and the particles are locked in place.
A liquid has no definite shape but definite volume because the matter has melted or condensed allowing the particles to move but stay connected together.
A gas is matter that is above boiling point and the particles are free to move and are not held together.
Plasma is the state of matter at the center of high temperature flames where particles come apart.
We sometimes classify matter as metallic because of its properties.
Metals are: Very Dense Lustrous Malleable and ductile Conduct heat and electricity
Matter is often grouped because of its physical properties. Uses of matter often depends on its physical properties.
Section Two Chemical Properties and Changes
A chemical property is a trait that gives a substance the ability to undergo a change that results in a new substance.
A chemical change is the change in idenity of a substance due to its properties. Substances often have warning labels because of their properties.
Chemical changes happen all around us all of the time.
The important thing to remember is that chemical changes form new substances.
Chemical Changes Have Certain Indicators: Change in temperature Production of a gas Production of a solid Production of light Production of electricity Change in color
It is important to remember that chemical changes are not reverseable.
In many chemical reactions, mass seems to disappear. This is not so, it only changes forms.