Snow pH Variance in Relation to Conifer Proximity Study
Investigating the impact of conifer proximity on snow pH and melting rates, this study hypothesized that closer proximity to trees would result in lower pH levels and slower melting. By analyzing snow samples from varying distances around conifer trees and conducting experiments, the study confirmed the hypotheses. Statistical analysis showed significant differences in pH levels at different distances from the trees, supporting the initial claims. The study provides insights into how tree proximity affects snow characteristics.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Snow pH Variance in Association with Conifer Proximity By Johnathan Xu, Devin Schinski, Noah Willis, Max Fairon
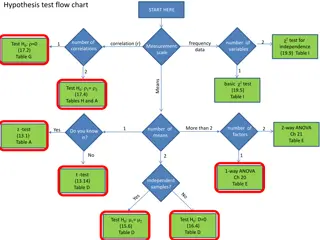
How does tree species and proximity of the sample sites to the tree base effect the snows pH and its ability to melt? Shortened to just study pH and melting, not species. Claims: The closer the sample site is to the tree, the lower sample shall have a lower pH (more acidic) and a slower melting rate. Trees are acidic and would alter snow pH The presence of H3O+ions will probably cause a slower melting rate than purer H2O. Desired Evidence: Snow samples from different trees, at consistent depths and distances from the tree. Research Question and Claims:
Materials 1. pH Meter (OHAUS): measures pH values in snow samples Ziplock bags: contains snow samples Tape Measure: measures the distance between each sample extraction site Shovel: digs out samples Probe: for extracting samples from depth Pot: used to melt snow samples 2. 3. 4. 5. 6.
Methods 1. 2. 1. Find appropriate conifer trees for data collection Measure out and dig 3 holes at 4ft, 8ft and 12ft from the tree trunk. Dig each hole 3 feet deep and take a labeled sample from each at 1.5 and 3 feet deep from the surface Melt snow in bag using hot water in a pot Measure and record pH Create a solution of 100 grams of tap water Create a solution of 80 g tap water mixed with 20 g lemon juice Freeze both solutions overnight Melt both solutions at the same time at room temperature, observe the speed at which each solution melts Infer how this would transfer to snow with varying pH 2. 3. 4. 3. 5. 4. 5.
Data Tree Sample pH Tree #1 #2 #3 #4 #5 4, 1.5 4, 3 8, 1.5 8, 3 12, 1.5 12, 3 7.0 6.6 5.8 6.7 6.5 7.3 6.3 5.9 6.5 6.3 7.1 6.3 5.9 5.7 6.1 5.8 6.6 6.2 5.5 6.3 6.1 6.4 5.8 5.5 6.2 6.0 6 5.8 6.3 5.9 Control Meadow pH Meadow pH #1 #2 0, 1.5 0, 3 5.4 5.8 5.8 6
Bar Graph Significance of a difference between 4ft and 8ft: p=0.0537 Significance of a difference between 4ft and 12ft: p=0.0318
Reasoning The p value from samples from 4 feet and 12 feet compared was less than 0.05. This significance indicated a trend of declining pH over the measured intervals. The branches of the fir trees studied were around 8-12 feet in length. There is almost no significant difference between 8 and 12 feet Using this information, we can reason that tree canopy had a direct effect on snow pH
Conclusion and Future Research From our experiment, we can conclude that snow pH has an inverse relationship to tree proximity. This can be connected to the ability of tree branches to shield the area closest to their trunks from acidic needle runoff. Our secondary experiment also showed that a solution of lower pH melted slower than a neutral solution of water. We can then infer that snow of a lower pH would also melt slower than snow of a more neutral pH. Further research would be required to test if pH did have a significant effect on snow melt rate. We would also perform more data collection on a variety of tree species to reinforce our current data.