Organometallic Chemistry III: Transition Metal Complexes and Homogeneous Catalysis
Explore the reactivity of transition metal complexes, including bond metatheses and various reactions. Learn about orbital considerations, synthesis, and spectroscopic properties of organometallic complexes. The course covers basics from AC1, focusing on ligands, electron counting, and MO diagrams. Assessment involves an oral exam describing a catalyzed reaction and proposing reaction mechanisms.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Inorganic Chemistry III: Organometallic chemistry and homogeneous catalysis 1
1. General Motivation: I was very interested in organometallic chemistry and wanted to understand more about the reactions we learned in OCII. Professors: Profs. Bezdek and Cop ret Format: Frontal lecture, blackboard. 2h lecture (Thursdays), 1h lecture or exercise on Friday 2
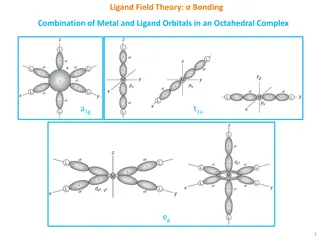
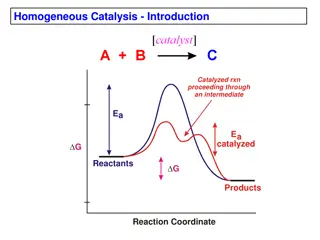
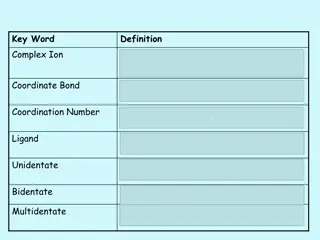
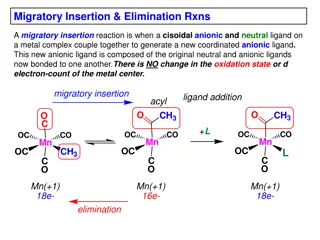
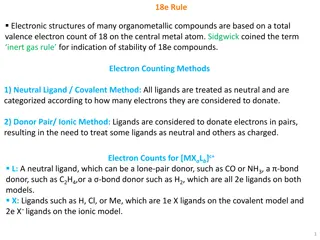
Content Reactivity of transition metal complexes: Bond metatheses, OA, RE, TM, MI, Beta-H Elim. Detailed mechanistic and orbital considerations. Trends based on identity of metal, ligands (sterics and electronics). Bonding in organometallic complexes (e.g. for carbonyl ligands, metallacycles etc.). Reactivity of carbonyl complexes, carbene, alkyl, olefin, imido etc., especially in the context of catalysis. Synthesis and spectroscopic properties of these complexes In my opinion, the basics of AC1 are prerequisite: Need to know the basics of ligands binding to metals, how to count electrons/oxidation states, basics of how to set up MO diagrams. Most of this is explained in ACIII again 3
2. Amount of work Exercise sessions every 2 weeks on average. Difficulty of exercises is on the harder side, so course 4-5 h per week. (I never actually did them tbh) During lernphase: Medium amount of work, repeating concepts and mostly solving problems and discussing them in a group! Concepts were not that hard to understand, especially since the basics are mostly known already. It s just a lot of material! 4
3. Unterlagen Blackboard only. Lecture notes by professors are provided Lecture notes are sufficient to follow the course, although Prof. Coper ts may be a bit confusing at times (so best visit the lecture) ;) No recordings! 5
4. Exam Only oral 30min Can vary, but on the easier side, both Profs are usually very friendly TM catalysed reaction is given, you need to describe the catalyst (electron count, ox state, ligands etc), give a proposed mechanism for the rxn (lots of time will be spent on each step) To prepare: Understand all of the content from the lectures, and the exercises. Know the elementary steps, what factors favor them and their mechanisms/transition states. Ideally, get a study group and discuss examples from literature (Mock exam style) 6
5. Subjektive Bewertung Qualit t (Vorlesung und Unterlagen): 11/10 Arbeitsaufwand um zu bestehen: 6/10 Unterhaltsamkeit: 8/10 Overall recommend if you want to know more about the reactions from the organometallic part of OCII. You don t learn many more reactions, but rather understand them on a deeper level. 7
Fragen? 8