Principles of Green Chemistry: Catalysis and Bio-Catalysis
The 9th Principle of Green Chemistry emphasizes the use of catalysis to improve reaction efficiency and reduce waste generation. Catalytic reagents enhance selectivity, reduce energy consumption, and minimize the need for stoichiometric reagents. Green catalysis, especially bio-catalysis using enzymes, offers a sustainable approach to chemical reactions by speeding up processes and increasing specificity.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
GREEN CHEMISTRY Twelve Principles of Green Chemistry By RITU MALIK Assistant Professor Rajdhani College University of Delhi
9th Principle of Green Chemistry Catalysis. Catalytic reagents as selective as possible are superior to stochiometric reagents Usually, the waste generation may be attributed to the traditional use of a stoichiometric amount of reagents.Therefore, greener approach will be to usage of catalysts methodologies instead of stochiometric methodologies,to improve the eficiency of a reaction and avoid waste generation. Catalysis enhance the efficiency of a reaction by lowering the energy intake, by avoiding the use of stoichiometric amount of reagents, and by greater product selectivity. This involves less energy, less feedstock and less waste. In addition, new innovative chemical reactions and unconventional solutions to traditional chemical challenges are being introduced. Examples are the oxidation and reduction (DIBAL-H as the hydride donor) reactions (Figure on next slide) Catalytic hydrogenation like the Noyori hydrogenationeliminates the need for stoichiometric reagents and in consequence decreases the amount of feedstock needed and the amount of waste generated (figure on next slide)
Comparison between stoichiometric and catalytic reduction.
Unknown Author is licensed under CC BY-SA This Photon by Unkown Author is licensed under CC BY Stochiometric versus catalytic reactions in the reduction of acetophenone: W Stochiometric versus catalytic reactions in the reduction of acetophenone: When acetophenone is reduced using stochiometric amounts of sodium borohydride, Atom economy is 81% and when the reaction is proceeded in the presence of catalyst, atom economy is 100% Hydrogen, Pd/C
Green Catalysis/Bio Catalysis-Enzymes act as catalysts In general, the catalysts used for the practice of green chemistry are usually Heterogenous catalyst as homogeneous catalyst may lead to the formation of byproducts as they intimately mix with the reagents involved in the chemical reactions. Green catalyst employed today, are the enzymes which carry out biochemical processes in the organisms. Biocatalysis refers to the use of living (biological) systems or their parts to speed up (catalyze) chemical reactions. Enzymes are the biological catalysts which regulate the rate at which the chemical reactions proceed. Enzymes increase the rate of biochemical reactions by factor ranging from 106 to 1012. The enzymes are highly specific and an average living cell contains more than 3000 different enzymes each catalyzing a specific reaction. Today, natural or modified enzymes to perform organic synthesis is termed chemoenzymatic synthesis; the reactions performed by the enzyme are classified as chemoenzymatic reactions
Advantages of Bio-catalysis Very highly enantioselective, chemoselective and regioselective. Very high transformation under under mild conditions Solvent often water Advantages of chemoenzymatic synthesis 1. Enzymes are environmentally benign, being completely degraded in the environment. 2. Most enzymes typically function under mild or biological conditions, which minimizes problems of undesired side-reactions such as decomposition, isomerization, racemization and rearrangement etc.. 3. Enzymes can be affixed on a solid support, demonstrating high stability and re-usability and can be used to conduct reactions in continuous mode in microreactors. 4. Advancements in protein engineering, mutagenesis and evolution; may lead non-natural reactivity of the enzymes. Variations may enhance reaction rate or catalyst turnover and allow a wider substrate range.
5. Enzymes exhibit extreme selectivity towards their substrates. Typically enzymes display three major types of selectivity: Chemo-selectivity: As an enzyme acts on a single type of functional group, other sensitive functionalities will not react to generate waste. As a result, biocatalytic reactions are cleaner; and labour, energy and time required for purification of product(s) from impurities formed through side reactions, is saved. Regioselectivity and diastereo-selectivity: As enzymes have 3-D structure, they are able to differentiate between functional groups that are chemically positioned in different regions of the substrate molecule. Enantioselectivity: As most of the enzymes are derived from L-amino acids, they are chiral catalysts. So, if substrate is chiral, it s chirality is recognized upon the formation of the enzyme-substrate complex. Hence, a prochiral substrate may be changed into an optically active product and two enantiomers of a racemic substrate may exhibit different rates. Due to above cited reasons, synthetic chemists are becoming more and more interested in bio catalysis. Bio catalysis has become significant due to its ability to synthesize enantiopure compounds that are chiral building blocks for Pharmaceutical drugs and agrochemicals.
Asymmetric Catalysis: Use of biocatalysis to obtain enantiopure compounds The use of biocatalysis to obtain enantiopure compounds can be divided into two different methods: Kinetic resolution of a racemic mixture Bio catalyzed asymmetric synthesis In kinetic resolution of a racemic mixture, the presence of a chiral compound(the enzyme) converts one of the stereoisomers of the reactant into its product at a greater reaction rate than for the other reactant stereoisomer. The stereochemical mixture is being transformed into a mixture of two different compounds, that can be separated by normal methods.
Biocatalyzed kinetic resolution is used in the purification of racemic mixtures of synthetic amino acids. For example, the Strecker Synthesis result in a mixture of R and S enantiomers. This mixture can be separated by acylating the amine using an anhydride followed by selective deacylation by enzyme (hog kidney acylase), which acts on only L enantiomer of only the L enantiomer. Thus, the two products are now separable by techniques such as chromatography.
The maximum yield in kinetic resolutions is 50%, as only one enantiomer of the racemic mixture reacts. These reactions must be stopped prior to equilibrium stage as after that, other enantiomer may form the product. If there is probability to carry out such resolutions under conditions where the two substrate- enantiomers are racemizing continuously, all substrate may be transformed into enantiopure product. This is termed as dynamic resolution. In bio catalyzed asymmetric synthesis, a non-chiral unit becomes chiral such that the different possible stereoisomers are formed in different quantities. The chirality is introduced into the substrate by impact of enzyme, which is chiral. Yeast is a biocatalyst for the enantioselective reduction of ketones.
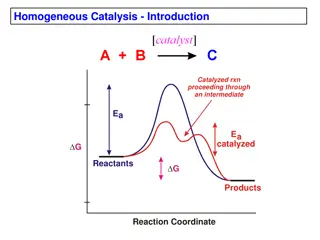
Comparison of Homogeneous Catalysis and Heterogenous Catalysis Homogeneous Catalysis In this type of catalysis, the catalyst is present in the same phase as the reactants Homogeneous Catalyst is evenly distributed throughout. The Homogeneous Catalysis can take place in gaseous or liquid phase. Examples of homogeneous catalysts in gas phase: 1. Oxidation of sulfur dioxide in the presence of nitric oxide as catalyst into sulfur trioxide 2. Decomposition of acetaldehyde in the presence of Iodine as catalyst 3. The reaction between carbon monoxide with oxygen in the presence of nitrogen monoxide as catalyst. 4. Reaction between moist sulfur dioxide and oxygen in the lead chamber process for the manufacture of sulfuric acid in the presence of Nitrogen Monoxide gas as catalyst.
Continued . Examples of homogeneous catalysis in liquid phase: Most of the reactions involving homogeneous catalysts in liquid phase involves acid or base as the catalyst 1. Acidic or Alkaline hydrolysis of an Ester.(Acid /Alkali as Catalyst) 2. Inversion of sucrose in acidic (aq) medium.(Acid as Catalyst) 3. Decomposition of hydrogen peroxide in the presence of chloride ions as catalyst. 4. Acidic conversion of acetone into diacetone alcohol. (acid as catalyst).
Heterogeneous catalysts Heterogeneous catalysts In this type of catalysis, the catalyst is in a different phase than that of the reactants. Usually, in most of the cases, the catalyst is solid while the reactants are either liquids or gases. As the reaction occurs by the contact of the reactants with the catalyst surface, this is also referred to as contact catalysis The catalyst is usually a metal or metal oxide. Heterogeneous catalysis may involve solid/ liquid /gaseous reactants.
Continued Examples of Heterogenous catalysis with gaseous reactants: 1. Reaction of sulfur dioxide and oxygen in the presence of platinum or vanadium pentoxide as catalyst (contact process). 2. Reaction of hydrogen and oxygen in the presence of platinum as catalyst 3. Hydrogenation of oils in the presence of nickel as catalyst. 4. Synthesis of petroleum by the reaction between carbon monoxide and hydrogen in the presence of Iron or cobalt or nickel as catalyst. 5. Manufacture of ammonia by Haber process involving reaction between nitrogen and hydrogen in the presence of Iron or molybdenum as catalyst.
Continued . Examples of Heterogenous Catalysis with liquid reactants: 1. Reaction between benzene and ethanoyl chloride to form phenyl methyl ketone in the presence of anhydrous aluminium chloride as catalyst 2. The decomposition of hydrogen peroxide(aq.) in the presence of manganese dioxide or platinum (in colloidal form) as catalyst. 3. The decomposition of hypochlorites (aq.) in the presence of catalyst oxides of cobalt and nickel as catalyst.
Photo Catalysis Photochemical reactions use the energy of photons of light or ultraviolet radiation to make the reactions happen. As photon is absorbed directly by molecule or functional group of a molecule, the electromagnetic radiation provides appropriate energy to the reaction medium and introduce energy into the reactant species without heating the medium. The photochemical energy is utilized to cause synthesis reactions to take place with high efficiency and with less generation of waste byproducts than non photochemical processes.
Example of Photochemical Acylation of Benzoquinone with an aldehyde-100% Atom Economy A reagent may may not absorb a photon directly to undergo a photochemical induced reaction. Sometimes, photochemical reactive species(example hydrogen peroxide) may be added which absorbs photons, generate reactive excited species or free radicals(hydroxyl radicals), that carry out the further additional reactions. ?2?2+ hv ??. + ??.
Photo-redox Biocatalysis Photo-redox Bio-catalysis Photo-redox catalysis depends upon light to produce free radical intermediates. These radical intermediates being achiral, generate racemic mixtures of product if there is a non-chiral external environment. Enzymes provide chiral environment and stabilize and form only one, enantiopure product. Photo-redox enabled bio catalysis fall into two categories: Internal coenzyme/cofactor photocatalyst External photocatalyst Common hydrogen atom transfer (HAT) cofactors (NADPH and Flavin) function as single electron transfer (SET) reagents. Although these are capable of HAT without irradiation, but their redox potentials enhance by nearly 2.0 V upon visible light irradiation, when paired with their respective enzymes (typically ene-reductases), leading to development of enantioselective reduction methods. For example medium sized lactams can be synthesized in the chiral environment of an ene-reductase through a reductive, Baldwin favored, radical cyclization terminated by enantio-selective HAT from NADPH. The other category of photo redox enabled bio catalysis use an external photocatalyst (PC). Many PCs with a wide range of redox potentials can be used. Rose bengal, and external PC, was used with an oxidoreductase to enantioselectivity deacylated medium sized alpha-acyl-ketones. Using an external PC has some disadvantages. For example, external PCs usually complicate reaction design as they react with both the bound and unbound substrate. If a reaction occurs between the unbound substrate and the PC, enantioselectivity is lost and other side reactions may occur.
10th Principle of Green Chemistry Design for Degradation: Chemical products should be designed so that at the end of their function they breakdown into innocuous degradation products and do not persist in the environment. The problem of non-degradation and accumulation of matter used during industrial processes is one of the serious problems faced by industries today as well as in the past. For example, in 1950 s tetra propylene benzene sulfonate (TPPS) was in use as a surfactant for laundry detergents and due to an incomplete degradation, it accumulated into the water. Later on, it was found that replacing the methyl branched chain of TPPS by a linear carbon chain (linear alkylbenzene sulfonate-LAS); decreases it s bio persistence (Fig. on next slide).
Continued.. Designing biodegradable materials and chemicals is not an easy task. The chemicals, that exhibit incomplete degradation such as halogenated moieties, branched chains, quaternary carbons, tertiary amines, and certain heterocycles should be avoided. While introducing functional groups such as esters or amides which are easily identified by enzymes may help in designing of environmental degradable products.This method was used in surface-active quaternary ammonium compounds for their usage as household fabric softeners. Until the 1990s, long chain ammonium salts such as di(hydrogenated)tallow dimethyl ammonium chloride (DHTDMAC) were released into the environment and was not completely biodegradable and even toxic, so hydrolyzable amide or ester linkages were introduced. Di(ethyl-ester) dimethyl ammonium chloride (DEEDMAC) was used instead of (DHTDMAC) and there was 70% increase in the biodegradability.
Integrating biodegradability in the design of surfactants.
11th Principle of Green Chemistry Real time analysis for Pollution Prevention. Analytical methodologies need to be further developed to allow for real time, in process monitoring and control prior to the formation of hazardous substances. The aim of green analytical chemist is to measure the chemicals without any waste generation, that is possible only if monitoring or measuring of chemicals can be done in situ (live analysis), so that any hazardous substance, if formed can be detected and its further formation can be controlled. But, till now, our analalytical strategies are not so advanced and we depend on pre-treatment of the sample or ex- situ analysis, that will lead to waste generation.
Advantages of real time analysis 1. Prevents accidents 2. Saves energy 3. Prevents the formation of by-products or waste generation, that further needs labour, energy and time for further purification. Problems faced in Analytical Chemistry: 1. Pre-treatment (extraction, separation or chemical modification) of the sample, that requires large amounts of solvents, thus generating lots of waste. If the solvents can not be avoided in an extraction step, benign alternatives such as Accelerated Solvent Extraction (ASE) or SCF extraction should be put into practice. 2. A signal acquisition step, that also requires solvents and leads to wastage of the sample itself. 3. Green Chemist must be aware that non-toxic and inert material should be used in the manufacture of analytical apparatus. Keeping this in view, nowadays; nanotubes/nanofibers electrodes are being used instead of conventional Mercury electrodes.
12th Principle of Green Chemistry Inherently Safer Chemistry for Accident Prevention. Substances and the form of a substance used in a chemical process should be chosen to minimize the potential for the chemical accidents, including releases, explosions and fires. Our environment is constantly exposed to dangerous substances and processes. In accordance with Chemical accident prevention and the clean air act amendments of 1990, we need to identify and assess all types of hazards (it s toxicity, physical hazards- explosivity, flammability and universal hazards), for designing of chemicals and processes to prevent accidents . All types of hazards whether it is toxicity, physical hazards such as explosivity or flammability, and global hazards should be acknowledged in the design of chemicals and processes in order to prevent accidents such as Bhopal or the Love Canal incident.A UCLA accident that occurred in January 2009, involving handling highly flammable butyllithium reagent, leading to the death of the research assistant. Such accidents are an alert to the scientific community. The hazardous and toxic chemicals we should be replaced by safer alternatives to prevent accidents.