Coordination Complexes and Transition Metals
Today's lecture covers transition elements, coordination complexes, ligand types, geometries, naming, isomers, and bonding in coordination complexes. Transition metals form coordination complexes with metal ions, ligands, and counter ions. The types of ligands include monodentate and bidentate ligands, which influence the coordination complex formed. Examples and common geometries like linear, square planar, tetrahedral, and octahedral are discussed with visual representations.
Uploaded on Sep 26, 2024 | 1 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Announcements Today s Lecture Transition Elements (Ch. 24) Coordination Complexes Ligand types (last time) Geometries Naming Isomers Bonding in Coordination Complexes - Theory
Chapter 24 Transition Metals Coordination Complex Covered previously (to some degree as complex ions) in Chapter 16, but focus now is on molecular scale view Coordination complexes consist of: metal ion (typically same charge as will exist in water although stability of different oxidation states such as Fe2+vs. Fe3+can change) ligand(s) counter ions (not part of complex, but associated with complex ion)
Chapter 24 Transition Metals Coordination Complex cont. Both the metal (covered more later in the chapter) and ligand affect the type of coordination complex formed Types of ligands: monodentate (one metal ligand bond per ligand) bidentate (two metal ligand bonds per ligand so requires to parts of ligand capable of acting as Lewis bases)
Chapter 24 Transition Metals Coordination Complex cont. Examples: Ag(NH3)2+= [Ag(NH3)2]+ H3N-Ag-NH3 linear structure uncharged monodentate ligand Ni(C2O4)2- O O- O- O oxalate is a bidentate ligand and forms a square planar complex (view from above) Ni2+ O- O O- O
Chapter 24 Transition Metals Coordination Complex - Geometries and numbers of ligands Most Common Geometries: Linear (with two ligands) example: H3N-Ag-NH3 Square Planar (4 ligand bonds) example: [PtCl4]2- Tetrahedral (4 ligand bonds) example: [Zn(OH)4]2- Cl Pt2- Cl Cl Cl OH Zn OH HO OH
Chapter 24 Transition Metals Coordination Complex - Geometries and numbers of ligands Most Common Geometries: Octahedral (with six ligand bonds note octahedral refers to 8 sides, even though 6 corners) example: [Co(H2O)6]2+ H2O OH2 Co H2O H2O OH2 H2O
Chapter 24 Transition Metals Coordination Complex - Geometries and numbers of ligands Example Questions Cobalt(II) forms a complex with three bidentate oxalate ligands. What is the geometry? Mercury reacts with 4 I- ligands. What geometries are possible? 1 EDTA ligand forms an octahedral complex with Ni2+. EDTA is a _____ dentate ligand
Chapter 24 Transition Metals Coordination Complex - Geometries and numbers of ligands More Questions What is the metal oxidation state and number of ligands + ligand bonds for the following compounds: 1. Mg[HgCl4] 2. [Co(NH3)5Cl]NO3 3. Na2[Cu(ox)2] (ox = C2O42-)
Chapter 24 Transition Metals Coordination Complex Naming Compounds Naming ligands: Neutral ligands are given molecule names (e.g. ethylenediamine) except for: H2O = aqua NH3 = ammine CO = carbonyl Anionic ligands are changed from anion: -ide becomes -o -ate becomes -ato -ite becomes -ito
Chapter 24 Transition Metals Coordination Complex Naming Compounds Naming ligands: List names of ligands in alphabetical order before cation name Prefixes used to indicate number of ligands (di-, tri-, tetra-, penta-, hexa-) or bis- , tris- if ligand name already has prefix or for polydentate Metal names (Depends on complex charge): Cations (metal name same as in ionic compounds) Anions (metal or Latin root ending in ate)
Chapter 24 Transition Metals Coordination Complex Naming Compounds Naming ligands information for exam: Too much to expect you to know all naming rules listed Should know all 4th row elements plus d8 to d10 5th and 6th row elements Focus on main rules: ligands names plus 3 exceptions at top of guidelines, di- to hexa- prefixes, will give table of Latin roots (e.g. ferrate) if needed
Chapter 24 Transition Metals Coordination Complex Naming Compounds Examples: [Ag(NH3)2]+ = [Pt(ox)2]2- = [Fe(NH3)4Br2]Cl = Tetracyanozincate = Pentaaquabromonickel(II) = Sodium diaquatetrachlorovanadate(III) =
Chapter 24 Transition Metals Coordination Complex Isomers What are isomers? Have same formula but are somehow different Structural isomers Have different connections between atoms Examples: - [Fe(NH3)5Br]Cl vs. [Fe(NH3)5Cl]Br (switch of counter ion with binding ion) - :C N: ligands can bind at C (cyano) or N (isocyano) side
Chapter 24 Transition Metals Coordination Complex Isomers Stereoisomers Due to different neighboring ligands Examples: [Pt(Cl)2(Br)2]2- (square planar) Trans isomer like ligands apart Cis isomer like ligands together Br Br Pt Pt Cl Br Cl Cl Cl Br These will have slight differences in properties (cis has slight net dipole moment while trans does not)
Chapter 24 Transition Metals Coordination Complex Isomers Stereoisomers cont. For tetrahedral compounds, MX2Y2 have only one isomer (X is same distance to other X and other Ys) For octahedral compounds, MX4Y2, also has cis- trans- isomers (guess which is trans) X X Y Y X X M X Y M X M X Y Y X X X Y
Chapter 24 Transition Metals Coordination Complex Isomers Stereoisomers cont. For octahedral compounds, MX3Y3, also has fac- (for face) and mer- isomers (for meridinal) mer fac X Y Y Y Y M X Y M X Y X X X
Chapter 24 Transition Metals Coordination Complex Isomers Stereoisomers cont. The stereoisomers mentioned so are geometric isomers that will have different properties (even if only slight differences) Another class is optical isomers, which have (mostly) identical properties; except that each isomer will rotate light differently (and can interact differently with other chiral molecules) A test for an optical isomer is if its mirror image is non-superimposeable (unique)
Chapter 24 Transition Metals Coordination Complex Isomers Optical Isomers (examples) MX2Y2 two or one optical isomer? MABCD two or one optical isomer? Not the same if we line up A-M-D, B and C are reversed 120 rotation about Z axis gives back original structure mirror mirror X A A X M M M X M D Y D B B Y X Y C C Y
Chapter 24 Transition Metals Coordination Complex Isomers Questions 1. Which of the following ligands will have linkage isomers? a) NH3 b) CN- e) C2O42- 2. In what way is [Cr(NH3)5Br]Cl2 different from [Cr(NH3)5Cl]BrCl? 3. How many different isomers are present for the square planar compound [Pt(NH3)2ClBr]? c) H2O d) SCN-
Chapter 24 Transition Metals Coordination Complex Bonding Theory Valence Bond Theory and Crystal Field Theory For covalent bonds (valence bond theory) overlap occurs between atomic orbitals from each atom (e.g. 1s in H and sp3 hybrid orbitals in C in CH4) For coordination compounds, however, electrons come fully from ligands
Chapter 24 Transition Metals Coordination Complex Bonding Theory cont. For example, in [Zn(OH)4]2-, bonding orbitals can come from empty 4sp3 on Zn2+ and filled 2p orbitals on O. (All electrons from O) However, for square planar and octahedral complexes, non empty d orbitals play a role (hybrid orbitals must have d character)
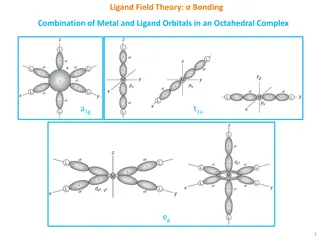
Chapter 24 Transition Metals Coordination Complex Bonding Theory cont. To understand how electrons in the d shells influence bonding, we must understand the shapes of d orbitals Two different classes of d orbitals occurs Off axes orbitals dxz dyz z z z y y y dxy lies in xy plane x x x
Chapter 24 Transition Metals Coordination Complex Bonding Theory cont. Two different classes of d orbitals occurs On axes orbitals dz^2 dx^2 y^2 z z y y x x