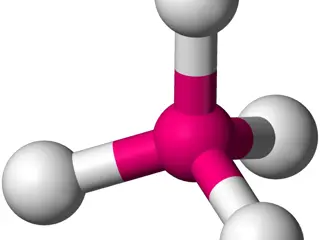
Coordination Numbers in Inorganic Compounds: Geometries and Structures
In inorganic coordination complexes, the coordination number refers to the number of atoms bonded to the central atom. Common geometries include octahedral, tetrahedral, and square planar, depending on the type and number of ligands. Transition metal complexes exhibit different coordination numbers and geometries. Examples include square pyramidal and trigonal bipyramidal arrangements for coordination number 5. The structure diversity can be seen in complexes such as [Cr(en)3][Ni(CN)5]·1.5H2O, showcasing multiple geometries in one crystal.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Coordination numbers of inorganic compounds (15-11-2018) In the field of inorganic coordination complexes it is the geometrical pattern formed by the atoms in the ligands that are bonded to the central atom in a molecule or a coordination complex. The geometrical arrangement will vary according to the number and type of ligands bonded to the metal centre, and to the coordination preference of the central atom, typically a metal in a coordination complex. The number of atoms bonded, (i.e. the number of -bonds between central atom and ligands) is termed the coordination number. The geometrical pattern can be described as a polyhedron where the vertices of the polyhedron are the centres of the coordinating atoms in the ligands. The coordination preference of a metal often varies with its oxidation state. The number of coordination bonds (coordination number) can vary from two as high as 20 in Th( 5-C5H5)4.One of the most common coordination geometries is octahedral, where six ligands are coordinated to the metal in a symmetrical distribution, leading to the formation of an octahedron if lines were drawn between the ligands. Other common coordination geometries are tetrahedral and square planar like complexes of AuCl4-, PtCl2Py2 and [Ni(en)2]2+ion. Here, we will focus on the common coordination numbers in most metal complexes and ions of transition elements which involve C.N=4,5 and 6, whereas the high coordination numbers like 7,8,9,10 and 12 are well- known in large atomic radium metals like tungsten (W), Re, OS, Nb, Mo,Ta,Zr and lanthanides such as U,Nb,Sm etc. Coordination Number 4 Two different geometries are possible. The tetrahedron is the more common while the square planar is found in particular with metal ions having a d8 electronicconfiguration.
Geometry of NiCl 2-ion (SP3hybridization 4 Geometry of dsp2 square planner complexes of Pt(II) complexes C.N=5. The five-coordinate symmetry have been shown in two expected geometries: Square pyramid, and trigonal bi pyramid as below: Square pyramid, (C4v) with (d4s) hybridization Oxovanadium salts (Vanadyl, VO2+) often show square pyramidal geometry, for example, VO(acac)2. Note that the Vanadium(IV) can be considered coordinatively unsaturated and addition of pyridine leads to the formation of an octahedral complex. Trigonal Bipyramid, (D3h)
The structure of [Cr(en)3][Ni(CN)5] 1.5 H2O was reported in 1968 to be a remarkable example of a complex exhibiting both types of geometry in the same crystal. Geometry of CN=5 of dsp3 for [Mn(CO)5]-ion Geometry of [Ni(CN)5]3-ion (dSP3 hybridization)
Octahedral, (Oh) The most common geometry found for first row transition metal ions, including all aqua ions. In some cases distortions are observed and these can sometimes be explained in terms of the Jahn-Teller Theorem. Geometry of [Cu(NH3)6]SO4 complex of sp3d2hybridization Coordination Number 7 Three geometries are possible: Not very common for 1st row complexes and the energy difference between the structures seems small and distortions occur so that prediction of the closest "idealised" shape is generally difficult. Capped octahedron, (C3v) Capped trigonal prism, (C2v)
Pentagonal Bipyramid, (D5h) 3- Pentagonal bipyramid d3sp3 hybridization of ZrF7ion 2- 3 3 Trigonal prismatic TaF7 ion (d sp) Coordination Number8 Dodecahedron, (D2d) Cube, (Oh) Square antiprism, (D4d)
Hexagonal bipyramid, (D6h) 3- Geometry of TaF8 Geometry of [Nd(H2O)9]2+ion Handout about coordination compounds(lectures1,2,3)
1.Indicate the coordination number for the central metal atom in each of the following coordination compounds: (a) [Pt(H2O)2Br2] (b) [Pt(NH3)(py)(Cl)(Br)] (py = pyridine, C5H5N) (c) [Zn(NH3)2Cl2] (d) [Zn(NH3)(py)(Cl)(Br)] (e) [Ni(H2O)4Cl2] (f) [Fe(en)2(CN)2]+ (en = ethylenediamine, C2H8N2) 2.Give the coordination numbers and write the formulas for each of the following, including all isomers where appropriate: (a) tetrahydroxozincate(II) ion (tetrahedral) (b) hexacyanopalladate(IV) ion (c) dichloroaurate(I) ion (note that aurum is Latin for gold ) (d) diaminedichloridooplatinum(II) (e) potassium diaminetetrachlorochromate(III) (f) hexaaminecobalt(III) hexacyanidochromate(III) (g) dibromo bis(ethylenediamine) cobalt(III) nitrate 3.Give the coordination number for each metal ion in the following compounds: (a) [Co(CO3)3]3 (note that CO32 is bi dentate in this complex) (b) [Cu(NH3)4]2+ (c) [Co(NH3)4Br2]2(SO4)3 (d) [Pt(NH3)4][PtCl4]
(e) [Cr(en)3](NO3)3 (f) [Pd(NH3)2Br2] (square planar) (g) K3[Cu(Cl)5] (h) [Zn(NH3)2Cl2] 1. Sketch the structures of the following complexes. Indicate any cis, trans, and optical isomers. (a) [Pt(H2O)2Br2] (square planar) 2. Draw diagrams for any cis, trans, and optical isomers th t could exist for the following (en is ethylenediamine): (a) [Co(en)2(NO2)Cl]+ (b) [Co(en)2Cl2]+ (c) [Pt(NH3)2Cl4] (d) [Cr(en)3]3+ (e) [Pt(NH3)2Cl2] 3. Name each of the compounds or ions given in Exercise 3, including the oxidation state of the metal. 4. Name each of the compounds or ions given in Exercise 5. 5. Specify whether the following complexes have isomers. (a) tetrahedral [Ni(CO)2(Cl)2] (b) trigonalbipyramidal [Mn(CO)4NO] (c) [Pt(en)2Cl2]Cl2 6. Predict whether the carbonate ligand carbonate (CO 2-) will coordinate to a metal center as a uni dentate, bi dentate, or tridentate ligand. 7. Draw the geometric, linkage, and ionization isomers for K3[Co(SCN)4(gly)2] 3