Ligand Field Theory in Octahedral Complexes
Ligand Field Theory explains the bonding interactions between metal and ligand orbitals in octahedral complexes. This theory involves the combination of metal and ligand orbitals to form molecular orbitals, leading to specific electronic configurations. The overlap of metal and ligand group orbitals results in the formation of different types of molecular orbitals, influencing the nature of bonding interactions in the complex. Various types of interactions, such as p-d, d-d, and d-*, play crucial roles in determining the stability and properties of these complexes.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
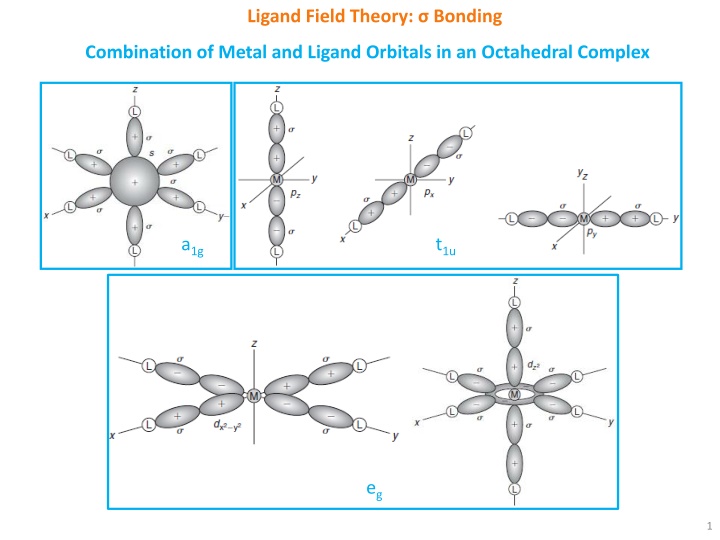
Ligand Field Theory: Bonding Combination of Metal and Ligand Orbitals in an Octahedral Complex a1g t1u eg 1
Ligand Field Theory: Bonding Overlap of metal orbitals and linear combination of ligand group orbitals (LGOs) leads to the formation of molecular orbitals (MOs). s orbitals transforms as a1g, set of p orbitals as t1u, whereas five d orbitals lose their degeneracy to form eg (dz2 and dx2-y2) and t2g (dxy, dxz and dyz). Spherical a1g orbitals are capable to overlap with ligand group orbitals (LGOs) on all axes. t1u and eg sets have lobes along bond directions and participate in bonding. However, t2g set have lobes directed between the bonding axis and thus will yield no overlap with ligand orbitals. Electronic configuration for [Co(NH3)6]3+: a1g2t1u6eg4t2g6 Electronic configuration for [CoF6]3-: a1g2t1u6eg4t2g4eg*2 2
Ligand Field Theory: Bonding MO Diagram for Octahedral ML6 Complex a1g = s t1u = px, py, pz t2g = dxy, dyz, dzx eg = dx2-y2, dz2 [Co(NH3)6]3+: a1g2t1u6eg4t2g6 3
Ligand Field Theory: Bonding Metal t2g orbitals, which are non-bonding in presence of -donor ligands, can form -bonds with ligand p , d , d * and d * orbitals. Pi-overlap with a metal t2g (dxy) orbital with ligand t2g LGOs Types of Interactions: p -d d -d d - * d - * 4
Ligand Field Theory: Bonding Types of Interactions Type Description Ligand Examples RO-, RS-, O2-, F-, Cl-, Br-, I-, R2N- ( -donors) p -d Donation of electrons from the filled p-orbitals of the ligand to the empty d-orbitals of the metal d -d Donation of electrons from filled d-orbitals of the metal to the empty d-orbitals of the ligand R3P, R3As, R2S ( -acceptors) CO, CN-, NO2-, ethylene ( -acceptors) d - * Donation of electrons from filled d-orbitals of the metal to the empty - antibonding orbitals ( *) of the ligand. d - * Donation of electrons from filled d-orbitals of the metal to the empty -antibonding orbitals ( *) of the ligand H2, alkane 5
Ligand Field Theory: Bonding MO Diagram for Octahedral Complex with -Donor Ligands [CoF6]3- 6
Ligand Field Theory: Bonding MO Diagram for Octahedral Complex with -Acceptor Ligands [Cr(CO)6] 7
Ligand Field Theory: Bonding Simplified picture of how -acceptor and -donor interactions affect MO diagram Only frontier orbitals are shown ( *) ( *) ( *) (t2g) ( *) ( ) ( ) [CoF6]3- -donor ligands decrease o -acceptors ligands increase o 8
Ligand Field Theory: Bonding -Donor Ligands: These ligands (e.g., F-) are more electronegative than metal and has filled p orbitals. Hence, the orbitals are lower in energy than the metal d orbitals. The 3 t2g metal orbitals and 3 low-lying, filled ligand orbitals form 3 bonding MOs and 3 antibonding MOs. The electrons from the ligand p orbitals will fill the bonding -orbitals. The electrons from the metal d orbitals will be present in the * orbitals. The eg* orbitals are not affected. The previously nonbonding metal t2g orbitals become antibonding and hence are raised in energy. As a result, o decreases. This is the reason for halides being weak ligands in spectrochemical series in spite of their negative charges. -Acceptor Ligands: These ligands are less electronegative than metal and has empty orbitals (d orbitals for PR3, * orbitals for CO). Hence, the orbitals are higher in energy than the metal d orbitals. The 3 t2g metal orbitals and 3 high-lying, empty ligand orbitals form 3 bonding MOs and 3 antibonding MOs. The electrons from the metal d orbitals will fill the bonding -orbitals. The eg* orbitals remain empty and they are not affected. The previously nonbonding metal t2g orbitals become bonding and hence are lowered in energy. As a result, o increases. This is the reason for PR3 and CO being strong ligands in spectrochemical series, although they are neutral ligands. 9
Ligand Field Theory: Bonding Explanation for the sequence of ligands within the spectrochemical series CO > CN- > PPh3 > NO2-> en > NH3> H2O> OH-> F-> Cl- > Br- > I- strong ligands weak ligands no suitable p-orbitals exclusively -donor (no effect) filled p-orbitals empty d or *-orbitals filled p-orbitals -donor weak donor -acceptor 10