Kinetic Reactions of SiO2 in Fluid Over Time
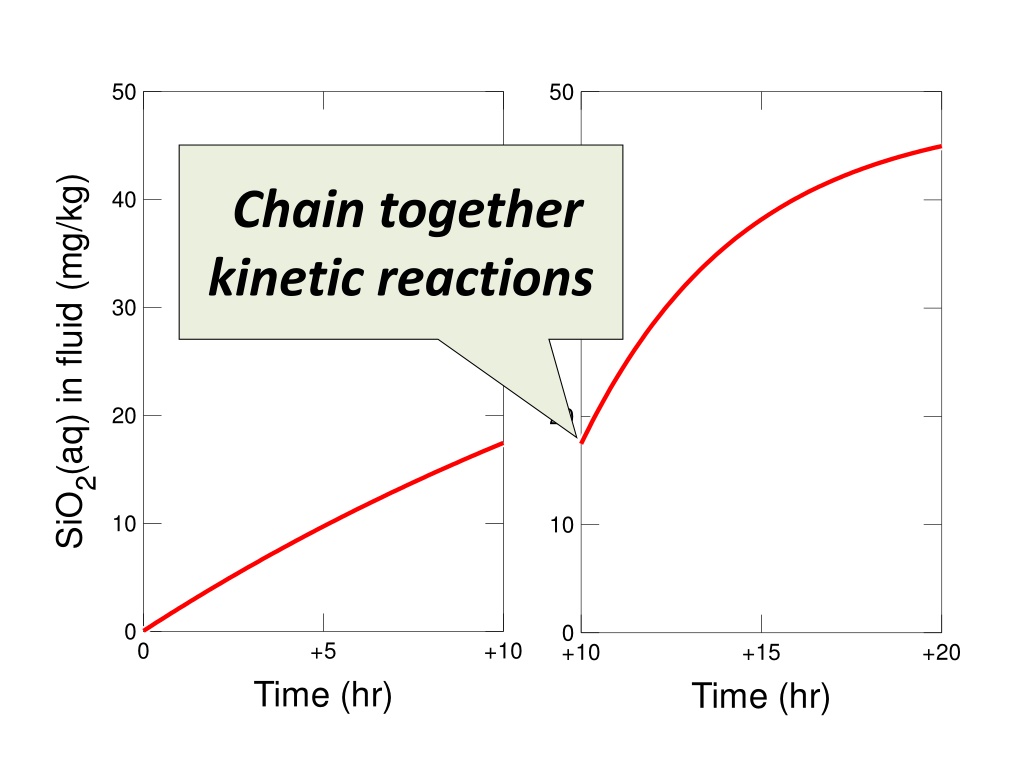
Experiment data illustrating the kinetic reactions of SiO2 in a fluid environment over time, showcasing the changes in SiO2 concentration. The graph depicts the chain reactions involving SiO2 concentrations and their evolution in the fluid medium as time progresses. The kinetics of SiO2 and its dynamic behavior can be observed through the data points provided. Understanding the progression of SiO2 in fluid systems is crucial for various scientific and industrial applications.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
50 50 SiO2(aq) in fluid (mg/kg) SiO2(aq) in fluid (mg/kg) Chain together kinetic reactions 40 40 30 30 20 20 10 10 0 0 0 +5 +10 +10 +15 +20 Time (hr) Time (hr)
Kinetic reaction paths can be chained together using the improved pickup command. Rate law for Quartz dissolution Initial mass, rate constant Run Go traces the path
Run Pickup System Entire resumes from the end of the last run SiO2 concentration retained from endpoint Run starts at previous endpoint Quartz mass retained from endpoint User can increase rate constant to reflect reaction promoter Run Go traces the second path
Endpoint concentration used as starting point for new calculation
The promoter added after 10 hours increases the rate at which Quartz dissolves into the fluid.