Overview of Serious Adverse Reactions and Transfusion Events
This data compilation covers the reporting trends, breakdown of reports, components issued, and specific types of adverse transfusion reactions experienced within the National Healthcare Organization (NHO) from 2019 to 2022. The information includes statistics on Serious Adverse Events (SAE), Serious Adverse Reactions (SAR), Near Miss incidents, and Acute Hemolytic Transfusion Reactions (AHTR). Additionally, it details Acute Transfusion Reactions, Febrile Reactions, and the components issued by the NHO during the same period.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
NHO Serious Adverse Reactions 2022 Joanne Scanlon Oct 2023
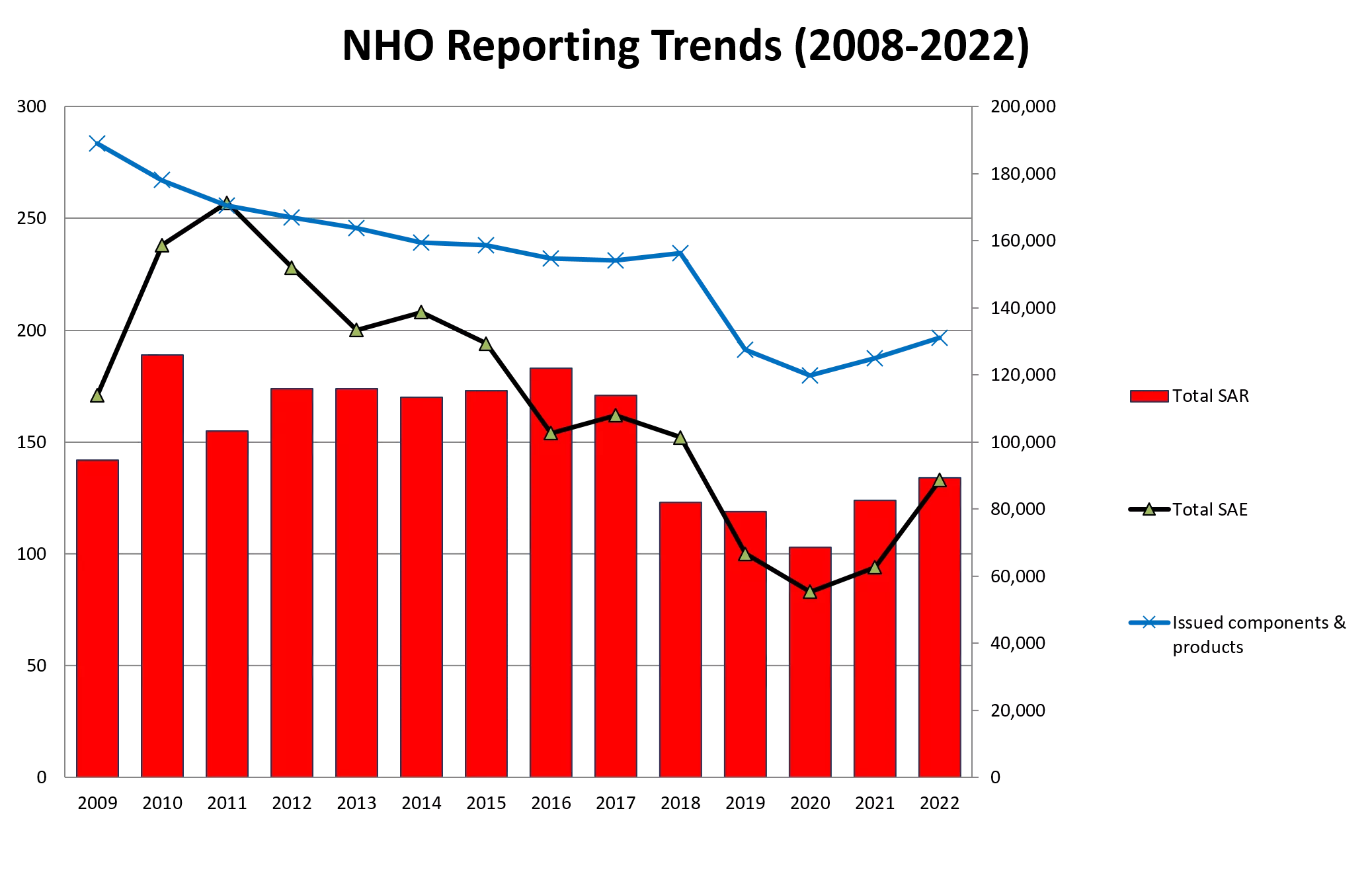
NHO Reporting Trends (2008-2022) 300 200,000 180,000 250 160,000 140,000 200 120,000 Total SAR 150 100,000 80,000 Total SAE 100 60,000 Issued components & products 40,000 50 20,000 0 0 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 2021 2022
Breakdown of reports received by the NHO (2019-2022) Report classification 2019 (n=332) 2020 (n=313) 2021 (n=322) 2022 (n=372) SAE 100 83 102 133 SAR 135 118 133 134 WBIT 54 77 56 57 Near Miss 43 35 31 48 The Breakdown of reports received by the NHO from 2019-2022
Components issued 2019 2022* RCC 122,582 Platelets 21,237 Other 163 Total Number of components issued 2019 Total Number of components issued 2020 113,766 21,049 92 Total Number of components issued 2021 122,762 22,738 109 Total Number of components issued 2022 122,899 22,907 117 *Figures from NHO ANSARE (Subject to Change)
Summary of Data 2022 45 40 35 30 25 20 15 10 5 0
Acute Hemolytic Transfusion Reactions (AHTR) AHTR is defined as a reaction occurring within 24 hours of a transfusion where clinical and/or laboratory features of haemolysis are present (ISBT definition). Acute haemolysis may be caused by ABO incompatibility, other antigen incompatibility or to non-immunological factors.
Acute Transfusion Reactions Acute Transfusion Reactions (n=82) Immunological Haemolysis due to ABO incompatibility 0 Acute transfusion reactions (ATRs) are defined as those occurring within 24 h of the administration of blood or blood components Febrile Non Haemolytic Transfusion Reaction 41 Anaphylaxis/Hypersensitivity 30 Hypotensive Transfusion Reactions Unclassified Reaction 4 7
Febrile Reactions (n=36) Components Findings 41 Reports received 36 Reports accepted 15 Reports Mandatory RCC + Pooled platelets RCC Pooled Platelets Demographics Neonate (< 28 days) : 1 1-4 yr: 2 Child (5-11 years): 1 12-17 yr: 2 18-30 yr: 2 31-50 yr: 5 51-70 yr: 11 70+: 12 Apheresis Platelets Multiple Compnenets Clinical Outcome Complete Recovery: 31 Minor Sequelae: 3 Death: 2 (unrelated to transfusion)
Febrile Reactions Interventions Investigations Antipyretics Bact screening of unit: 23 Bact screening of pt: 24 Bact screening of both pt and unit: 18 Diuretics Steroids Antihistamine Oxygen Fluids Antibiotics 0 5 10 15 20 25 15 Patients treated with 1 or more of above interventions
Anaphylaxis/hypersensitivity (n=27) Number of reports received: n =30 No. of reports accepted: 27 Clinical Outcome: Deaths: 0 Complete Recovery: 26 Minor Sequelae: 1 Demographic Data 11 16 Adults: 37 Less than 4 Years: 0 Components: Red Cells n= 9 Apheresis Platelets n= 15 Pooled Platelets n=4 Plasma n= 1 Multiple Components n=2
Anaphylaxis/hypersensitivity (n=27) Investigations and Treatment IgA levels: 12 (all NAD) Bact screening unit: 8 Bact screening pt: 10 Bact screening both product and patient: 4 Fluids Oxygen Antihistamine Steroids Anti-pyretics Pressor Agents Nebulisers/Inhalers 17/27 treated with 1 or more of the above interventions
Case Study - AA Background 45 yr old female patient with severe iron deficiency anaemia Hb: 5.5g/dl, transfused RCC x 1 2 hours post commencement of transfusion pt developed: Hypertension Tachycardia Increased RR Lip swelling
Case Study - AA Investigations IgA Levels: Normal Chest Xray: Normal Hb post transfusion: 5.1g/dl Bacterial screening of both patient and unit: Negative Intervention Transfusion completed Pt treated with Antihistamines and Steroids Clinical Outcome: Minor Sequelae Future Requirements for this pt: Pre-med of steroids and anti- pyretics
All patients should be transfused in clinical areas where they can be directly observed and where staff are trained in the administration of blood components and the management of transfused patients, including the emergency treatment of anaphylaxis. (BSH 2023) The recognition and immediate management of ATR should be incorporated into local transfusion policies and there should be mandatory transfusion training requirements for all clinical and laboratory staff involved in the transfusion process. (BSH 2023)
Hypotensive Transfusion Reactions (n=3) The NHO received 3 reaction under the category of Hypotensive transfusion reaction Platelet Apheresis Case Age Group Gender Imputability Red Cells Elderly (70+) 1 Female Possible Yes Elderly (51 - 70 years) Yes 2 Male Likely/Probable Elderly (70+) Yes 3 Female Possible
Hypotensive Transfusion Reactions (n=3) Case 1: 88 yr old patient admitted with PR Bleed- fresh blood and melena. Patient received RCC x 1. 28 minutes into transfusion BP dropped from 135/69mmhg to 97/41mmhg. Patient treated with IV fluids and made a complete recovery within 100 minutes. Case 2: 63 yr. old patient with AML, Plt count 19 received Apheresis plts x 1. BP dropped from 139/83 mmhg to 70/49 mmhg. Patient treated with IV fluids and made a complete recovery within 70 minutes. Case 3:74 yr old patient with Lung Ca (Covid +) received RCC x 1. 1 hour into transfusion BP dropped from 106/68 mmhg to 77/49 mmhg. Patient treated with IV fluids and Oxygen
Key Messages Assessment of signs and symptoms when a reaction occurs is critical in the provision of treatment. Education in relation to this is fundamental For febrile reactions paracetamol is key treatment If anaphylaxis is suspected adrenaline should be available Steroids may take several hours to have an affect Pooled platelets in PAS/HLA matched platelets should be considered with patients with recurring reactions
Unclassified Reaction (n=6) Definition Unclassified SAR is the occurrence of an adverse symptom / sign with no risk factor other than the transfusion and which on its own does not allow the reaction to be classified within the defined categories of SAR. Findings Reports of 6 unclassified reactions were received Commentary Reporting establishments are advised to continue reporting cases with unusual symptoms or those reactions which may not fit into the criteria already in place
Unclassified Serious Adverse Reactions 2022(n=6) Component Transfused Age Profile Imputability Description Unclassified reaction. Patients symptoms included Hypertension, Tachycardia, Chills and Rigors, Dyspnoea (no drop in O2 Sats) and Cyanosis. Patient received O2 Elderly (70+) Likely / Probable Case 1 Platelets Apheresis Adolescent (12-17 years) Patient had drop in O2 sats only, required oxygen and overnight admission Case 2 Red Cells Possible Unclassified reaction. Patients symptoms included Pyrexia, Rigors and Desaturation to 84%. Patient received O2 (10L) and Anti- Pyretics, Child (5-11 years) Possible Case 3 Red Cells Likely / Probable Patients symptoms included Hypotension, complaints of all-over pain. Pain eased when transfusion stopped, no intervention required. Adult (31-50 years) Case 4 Red Cells Originally classified as non-mandatory TACO, however patient received DIURETICS Elderly (70+) Possible Case 5 Red Cells Initially reported as non-immunological haemolysis, however no evidence of haemolysis Elderly (70+) Case 6 Red Cells Possible
Delayed Transfusion Reactions: Immunological Haemolysis due to other allo- antibody (delayed n=5) 7 Reports received 5 Reports accepted Age Range Adult (31-50 years) : n=2 Adult (51 - 70 years) : n=1 Elderly (70+) : n=2 Clinical Outcome All reactions reported a Clinical Outcome of complete recovery
Timeframe for developing antibody Case No. Antibody identified Reaction caused by error Age Gender Findings Outcome LDH, Bilirubin, + DAT Elderly 70+ years Complete Recovery 1 Male Anti S No 13 Days LDH, + DAT Anti Jka Complete Recovery Adult (31-50 years) 2 Female No 6 Days Hb, LDH, Bilirubin Elderly 70+ years Complete Recovery 3 Male Anti E No 11 Days +DAT, Bilirubin Complete Recovery Adult (31- 50 years) 4 Female None No 4 Days LDH, +DAT, Bilirubin Adult (51 - 70 years) Complete Recovery 5 Female Anti E No 7 Days
Case Study DHTR Background 42 yr old pt with SCC requiring RCC every 3/52 HB 9.1mm/hg o/a as day patient Patient readmitted 5 days later with myalgia, pain to arms and legs, Haematuria, blood in stool. Developed Acute chest syndrome complicated by Hyperhaemolysis
Case Study DHTR 6 Days later: Patient readmitted Hb now 7.2mm/hg Haemoglobin TBIL Other Day 1 (Pre- Transfusion) 9.1 mm/hg Day 2 (Transfused) 10 mm/hg Day 6 7.2 mm/hg 120.4 Chest Xray changes, Falling O2 sats Dyspnoea, DAT Positive (pre and post transfusion)
Case Study DHTR Treatment Oxygen, Antibiotics, Antihistamine, Fluids, IVIG Complete Recovery from transfusion Review Classical Hyper haemolysis syndrome linked to transfusion in sickle cell disease patients. Serological investigations not too informative (often the case). No red Cell antibody identified Immune mediated haemolysis and probable hyper haemolysis
Delayed Transfusion Reactions Recommendations Lifesaving transfusion should not be withheld due to a history of alloantibodies. Robust methods of recording patients antibody history should be developed and supported with patient education
Transfusion Transmitted Infection (n=1) Serious Adverse Reaction Platelets Apheresis Platelets Pooled Age Gender Imputability Red Cells Transfusion transmitted viral infection (HCV) Transfusion transmitted viral infection (HBV) Adult (51 - 70 years) 1 Male Possible RCC X 3 Elderly 70+ years 2 Male Unlikely RCC X 2
Possible Transfusion transmitted viral infection(HCV) Background NHO informed June 2022 of patient having been transfused in 1992 following Opthalmic/Facial injury Patient now HCV Positive Reaction previously reported to RTU in 2019, however with very minimal information June 2022: QC Dept. in IBTS aware and HPRA informed
Possible Transfusion transmitted viral infection (HCV) 2019: Trace back not performed due to limited information available 2022: Unit numbers received, however only 3 out of the 6 digits received 2022: Trace back unable to proceed Many efforts by all involved to retrieve information, however full 6-digit numbers unavailable June 2022: Case accepted as possible transfusion transmitted infection
STTI Recommendations Inform NHO, IBTS Quality Department, IBTS Consultant on call or Medical Scientist on call ASAP in cases of STTI to protect the blood supply Where a recall involves blood components which have been transfused, hospitals should have a robust system in place which should include a review of the patient.
TACO Points to note The NHO continue to collect reports of TACO where patients exhibit clinical signs and symptoms of overload following transfusion and do not meet the strict criteria of ISBT Definition The NHO following review and discussion will make a decision if the reaction fits the ISBT Definition criteria
Transfusion Associated Circulatory Overload (TACO) n=38 Findings 40 Reports received 38 Accepted 38 Mandatory Demographics Adolescent (12-17 years) :1 31-50 yr: 1 51-70 yr: 9 70+: 27 Components RCC: 35 Platelets (Apheresis): 2 Multiple Components :1 Clinical Outcome Complete Recovery: 25 Minor Sequelae: 8 Serious Sequelae: 1 Death: 4 (unrelated to transfusion)
Clinical Features TACO Resp Distress Fluid Overload Cardiac Problems Deteriorating Renal Function Other Electrolyte Imbalance
Interventions (TACO) Other Anti-Pyretics Pressor Agents Antihistamine Steroids Nebulisers/Inhalers Antibiotics Oxygen Diuretics 0 5 10 15 20 25 30 35 40 35 patients receiving 1/more of the above interventions
TACO as a result of an error 3 out of the total 38 accepted TACO cases reported occurred as a result of an error with Human Failure been identified as the main cause of error Case Number Age Human Failure System Failure Error Cause Imputability Adult (51 - 70 years) Patient with pre-existing cardiac problems, unit transfused too quickly 1 Certain Yes No Adolesce nt (12-17 years) No Patients IV input in the previous 24 hrs not monitored 2 Possible Yes Adult 31- 50 yr (years) Certain No At risk patient prescribed and transfused 2 units RCC over 1hr each 3 Yes
Transfusion Associated circulatory Overload (TACO) 19/38 patients transfused with a reason categorised as anaemia 29/38 patients transfused with a Hb between 7-8g/dL(WHO defined Chronic anaemia as lower than 7-8g/dL in adults) 29 patients developed TACO following 1 component transfused 9 patients developed TACO following transfusion of 1 or more components
Case study TACO 13 yr old Haematology patient with AML Hb 5.5g/dl pre transfusion Pt prescribed RCC x 1 Pre transfusion obs : HR: 82bpm, BP:127/71mmHg, Temp: 38 Patient on 2L O2 pre transfusion PEWS: 4 Previous 24 hours Plasma Units x 4 Platelets x 3 RCC x 1 Novoseven x 1 IV Fluids
Case study TACO Symptoms (1 hour 25 mins into transfusion) Hypertension 137/80 mm/Hg Tachycardia 82 100 bpm Pyrexia: 40.3 Dyspnoea O2 sats 92% Chest Xray changes Intervention Transfusion stopped Positive Fluid balance noted Diuretics administered O2 increased Patient observed closely
Case study TACO Review Monitoring error Patient in a positive fluid balance pre- transfusion, transfusion could have commenced later NHO enquired as to perhaps this reaction was as a result of a SAE Reinforced the need for a TACO checklist
Pulmonary Reports Year on Year 45 40 35 30 25 TACO Non-TACO 20 15 10 5 0 2013 2014 2015 2016 2017 2018 2019 2020 2021 2022
Appropriate management of anaemia and making safe transfusion decisions Ensure decision to transfuse blood is made in careful consideration with risks and benefits Early detection and treatment of preoperative anaemia in patients with high probability of transfusion intra/post-op Minimising blood loss and anaemia Risk of transfusion No single approach is effective for all patients (drug,device,technique)
Transfusion Associated Dyspnoea (TAD) TAD (ISBT definition) TAD is characterized by respiratory distress within 24 hours of transfusion that do not meet the criteria of TRALI, TACO, or allergic reaction. Respiratory distress should not be explained by the patient s underlying condition or any other cause Findings The NHO received 2 reports of TAD for the 2022 reporting period.
Transfusion Associated Dyspnoea (TAD) Case No. Age Gender Imputability Components Comments Dyspnoea approx.110 mins into transfusion. SAR as result of SAE. Transfused based on inaccurate HB result Elderly (70+) Likely / Probable 1 Male RCC Adult (51-70 years) Dyspnoea post transfusion. Patient desaturated to 85% 2 Female Possible RCC
Respiratory Complications of Transfusion Points to note There is still much work that needs to be done to understand cases reported Patients generally have multiple co- morbidities Risk factors need to be identified and acted on
Mortality and morbidity data by category 2022 Death (unrelated to transfusion) Death probably related Death possibly related Major Sequlae Minor Sequlae Complete Recovery Unknown 1 26 Anaphylaxis/hypersensitivity (AA) 1 Immunological haemolysis due to other allo-antibody (Acute < 24 hrs) Immunological haemolysis due to other allo-antibody (Delayed > 24 hrs) 5 3 Hypotensive Transfusion Reaction OSR - Febrile Non Haemolytic Transfusion Reaction OSR - Transfusion Associated Circulatory Overload (TACO) OSR - Transfusion Associated Dyspnoea 2 3 31 4 1 8 25 2 6 OSR - Unclassified SAR Transfusion transmitted viral infection - Other 1 6 0 0 1 12 99 1 Total
Future plans for NHO Online reporting Handbook update Resourcing 1 FTE Haemovigilance Officer NHO Medical Director eLearning Continued collaboration with all stakeholders
Acknowledgements NHO Team Prof Hervig Vigilant reporters and hospital staff RTU Team IBTS Quality team For further information please find us on www.giveblood.ie