Evaporation and Condensation Through Water Molecules
Exploring the water cycle and states of matter, this lesson delves into how ideas about water molecules aid in explaining evaporation and condensation. Through diagrams, simulations, and real-life scenarios, students learn about the movement and changes of water molecules. By comparing their ideas with a scientific model, they deepen their understanding of these processes. Situations like a puddle evaporating under the sun and a cold soda can condensing on a hot day illustrate the role of energy and water molecules in state changes.
Uploaded on Sep 19, 2024 | 2 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
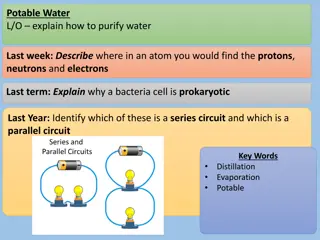
WATER CYCLE LESSON 4A How Can Ideas about Water Molecules Help Us Explain Evaporation and Condensation?
Review: States of Matter 1. What does this diagram show? 2. What state of matter do you think these water molecules are in? How do you know?
Review: States of Matter 1. What does this diagram show? 2. What state of matter do you think these water molecules are in? How do you know?
Todays Focus Question How can ideas about water molecules help us explain evaporation and condensation?
What Happens to Water Molecules? What do you think happens to water molecules during evaporation and condensation? How do you think they move?
Lets Find Out What Happens! Watch the simulation and then answer the questions on your handout about energy and water molecules. Link to simulation: https://phet.colorado.edu/en/simulation/states-of-matter
Comparing Our Ideas with the Simulation Which ideas on our chart does the scientists model support? Which ideas does the model NOT support? Explain your thinking.
Situation 1: A Puddle of Rainwater After a rainstorm, there is a puddle of water on the sidewalk, and the Sun is out. Photo courtesy of Pixabay.com 1. What change of state is going to occur? 2. Why will this change of state happen? Use the words energy and water molecules in your answer.
Situation 2: A Cold Soda Can On a hot day, you put a very cold can of soda on the table. Photo courtesy of Pixabay.com 1. What change of state is going to occur? 2. Why will this change of state happen? Use the words energy and water molecules in your answer.
Summary Statements Today s focus question: How can ideas about water molecules help us explain evaporation and condensation? Write a summary statement for the question assigned to you: 1. What happens to water molecules during evaporation? 2. What happens to water molecules during condensation?
Lets Summarize! How can ideas about water molecules help us explain evaporation and condensation? Key ideas: During evaporation, molecules in the liquid state gain heat energy, which makes them move faster and escape into the air as water vapor (a gas). During condensation, water-vapor molecules lose energy (cool), which makes them slow down and join together to form liquid-water droplets.
Next Time Tomorrow we ll think more about evaporation and condensation as we observe a demonstration of water changes in a system.