ATP Synthase: Enzyme for Energy Production
ATP synthase is a crucial enzyme responsible for generating ATP, the energy currency of cells. Explore its structure, location, discovery, and mechanism of ATP synthesis in prokaryotes and eukaryotes. Learn about the F0 and F1 portions, the role of subunits, and the intricate process of ATP production and hydrolysis.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
ATP SYNTHASE ARPITA BERA ARPITA BERA 6TH SEM , paper: C13T(unit 5) BOTANY(H) TAMRALIPTA MAHAVIDYALAYA TAMRALIPTA MAHAVIDYALAYA
INTRODUCTION ATP synthase is an enzyme that creates the energy storage molecule ATP i.e used as energy currency of cells for all organism. ATP generated by catalyzing the addition of an inorganic phosphate to ADP. ADP + Pi + H+ ATP + H20 + 3H+ It also known as F-ATPase and it also called molecular machine for its rotating subunit. ATP synthase utilizes the energy stored in this electrochemical gradient to drive nucleotide synthesis.
LOCATION In case of prokaryotes it s found in plasma membrane. In case of eukaryotes it is found in thylakoid membrane of chloroplast and inner membrane of mitochondria.
DISCOVERY Herman Kalckar(1937): ATP synthase is linked with cell respiration. Ephrain Racker(1961):Isolates the f1 part of the ATP synthase. Peter Mitchell(1964): Cell respiration loads to ph change inside and outsides the mitochondria. John E. Walker(1981): Determines the DNA sequence of the genes encoding the protein in ATP synthase. Walker et al. (1994): Determine the structure of the F1 part of the ATP synthase. Richard Cross, Wolfgang Junge & Masasuke Yoshide (1997): Demonstrated ATP synthase rotate during the synthesis and hydrolysis of ATP.
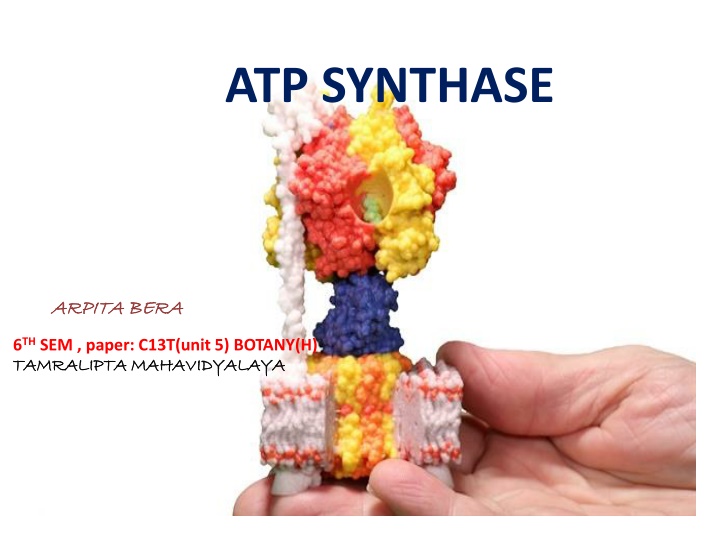
STRUCTURE Consist of two regions i.e F0 & F1. F0 portion is integral into inner membrane and F1 portion is located in matrix.
F0 Portion Water insoluble protein with eight subunits transmembrane ring. It consist of three main subunits a, b & 9 -14 copies of c varies species to species. Six c subunits make up the motor ring. Subunit b makes up a stalk connecting to f1 that prevent hexamer from rotating. Subunit a connects b to c ring. Proton pore present in each c ring. and a
F1 Portion F Hydrophilic and responsible for hydrolyzing ATP. Consist of , , , & subunit. Subunit and make a hexamer ( 3 3) with 6 binding sites. Three of them catalytically inactive & they binds ADP and others three subunits catalyze synthesis. , , , are part of rotational motor mechanism. subunit allows to go through conformational change that allow for ATP to be bound and released once synthesized. the ATP
Mechanism of ATP synthesis Three subunits assume 3 different conformation :- 1. - ADP 2. - ATP 3. - empty Given subunit starts in the -ADP conformation, which binds ADP & pi . Then change conformation, assuming the - ATP form that tightly binds ans stabilizes ATP. Finally the subunit changes to the - empty conformation which has very low affinity for ATP. another round of catalysis begins when the subunit again assume the - ADP form and binds ADP & pi. the conformational changes central to this mechanism are driven by the passage of proton through F0 portion. the streaming of proton through the F0 pore causes the cylindr of c subunits and attached subunits to rotate about the long axis of .
PHYSIOLOGICAL ROLE Creating energy in oxidative phosphorylation. To create a transmembrane proton gradiant. Generate ATP through pumps proton Hydrolysis ATP for ADP +pi and ATP equilibrium INHIBITOR Oligomycin and DCCD
REFERENCE .r Cross RL, Taiz L (January 1990). "Gene duplication as a means for altering H+/ATP ratios during the evolution of FOF1 ATPases and synthases". Junge W, Nelson N (June 2015). "ATP synthase". Annual Review of Biochemistry Stock D, Leslie AG, Walker JE (November 1999). "Molecular architecture of the rotary motor in ATP synthase".
Thank you Thank you